Translate this page into:
Atypical presentation of osteosarcoma: A case report

*Corresponding author: Chinky Patel, Department of Radiology, Le Bonheur Children’s Hospital, Memphis, United States. cpatel23@uthsc.edu
-
Received: ,
Accepted: ,
How to cite this article: Patel C, Sandhu PK. Atypical presentation of osteosarcoma: A case report. Indian J Musculoskelet Radiol. doi: 10.25259/IJMSR_52_2024
Abstract
We present a 4-year-old male who presented with right arm mass and deformity following a fall onto his forearm. On imaging, the entire right humerus was involved by an aggressive, infiltrating process with associated extraosseous masses. This was proven to be osteosarcoma (OS) on biopsy. This is a rare diagnosis in this age group. The imaging features are also non-characteristic for OS wherein the entire length of the long bone is involved. OS should be included in the differential for aggressive bone tumors in the pediatric age group regardless of the known bimodal age distribution.
Keywords
Aggressive bone tumor
Diaphyseal
Intraarticular
Osteoid matrix
Osteosarcoma
Transepiphyseal
INTRODUCTION
Osteosarcoma (OS) is a malignant tumor of connective tissue that produces osteoid matrix and variable amounts of cartilage matrix and fibrous tissue.[1] It is the third most common cancer in adolescents after leukemias and lymphomas.[2,3] There is a bimodal pattern of incidence, affecting the younger population under 20 and older population over the age of 60. The incidence is 1.4 times greater in males than in females.[4] OS most commonly involves the metaphysis of long bones, particularly the knee and shoulder.[5] The distal femur, proximal tibia, and proximal humerus are other common sites of involvement. While the exact cause remains elusive, genetic predisposition, radiation exposure, and certain chemotherapy agents are established risk factors.[6] The pathogenesis involves abnormal development and proliferation of primitive bone-forming cells, often exhibiting mutations in tumor suppressor genes such as TP53 and RB1.[6] Prognosis depends on various factors, with a 5-year survival rate of around 60–70% achievable through a combination of aggressive surgery, chemotherapy (including agents such as doxorubicin, cisplatin, and methotrexate), and sometimes radiotherapy.[7] Radiologists play a crucial role in diagnosis and treatment planning by utilizing imaging techniques such as X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) to assess tumor location, extent, and response to therapy.[6] We present a case of OS in a much younger age group involving the entire length of the humerus.
CASE REPORT
A 4-year-old male presented with right arm swelling and decreased range of motion following a fall. He did not have significant pain although there was a palpable mass with associated weakness on physical examination.
Radiographs of the right humerus and right shoulder were obtained [Figure 1], which demonstrated a diffuse moth-eaten appearance of the right humerus with a pathological fracture of the proximal diaphysis. Extensive spiculated periosteal reaction was noted with areas of periosteal elevation. The infiltrative process extended to involve the proximal and distal epiphyses of the humerus. The differential considerations included Ewing’s sarcoma, leukemia, and lymphoma. Osteomyelitis was felt to be unlikely given the extensive nature of the disease process and lack of infectious symptoms. Abdominal ultrasound and chest radiographs were negative for metastasis. MRI [Figures 2 and 3] confirmed a diffuse and aggressive infiltrative process of the entire right humerus replacing bone marrow, pathological fracture involving the proximal diaphysis, and asymmetrically thick circumferential rind of heterogeneously enhancing soft tissue mass and additional multiple nodular appearing soft tissue masses. There was transphyseal extension at the proximal end into the humeral head with suspicion of glenohumeral joint involvement, without joint effusion. The mass showed an abutment of the neurovascular bundle without definitive encasement. Survey of the right forearm was negative for skip lesions.
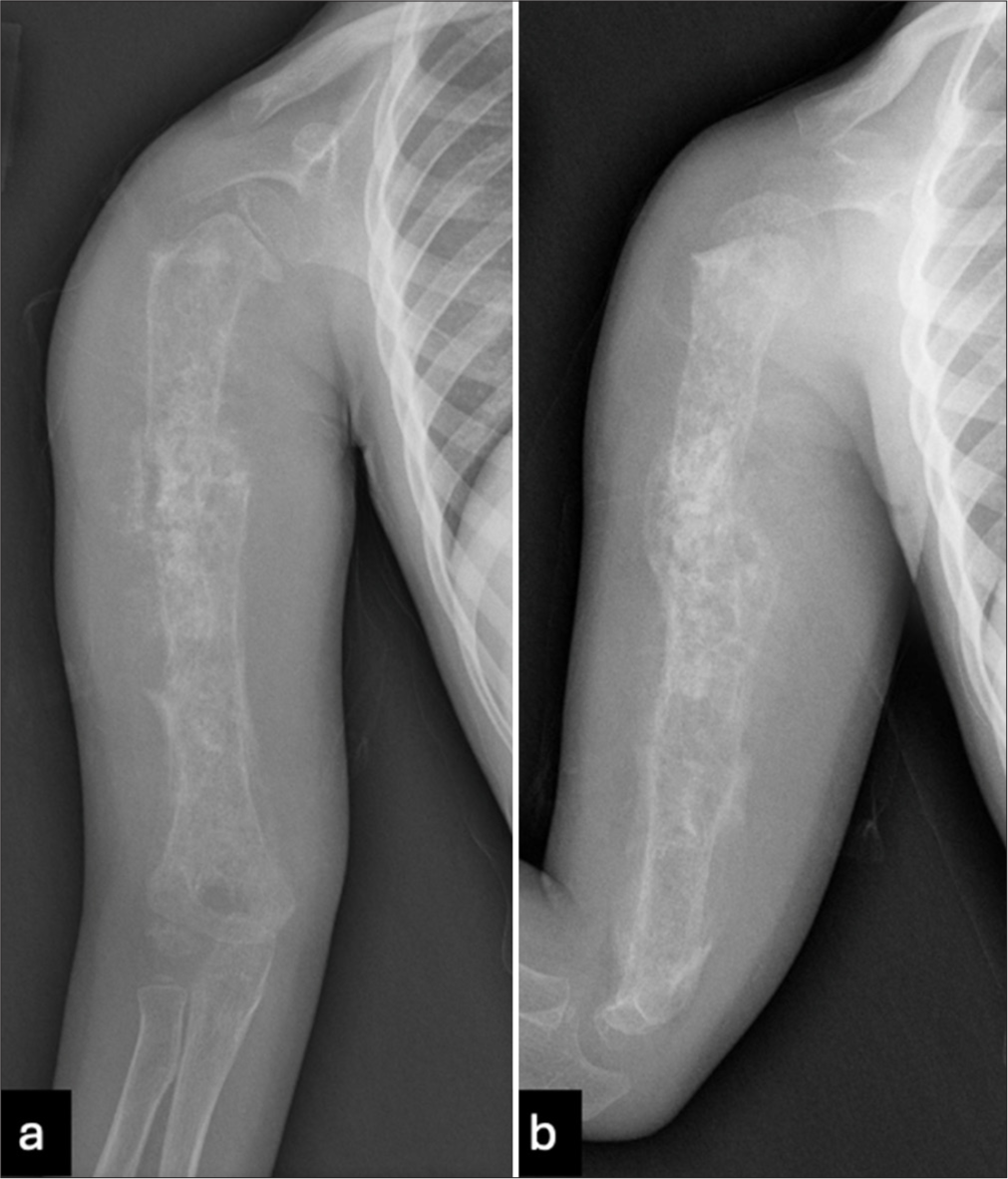
- (a) Frontal and (b) lateral radiographs of the right humerus show a diffuse moth-eaten appearance involving the entire humerus length from proximal to distal epiphyses. There is an associated pathological fracture of the proximal diaphysis. There is extensive spiculated periosteal reaction with areas of periosteal elevation.
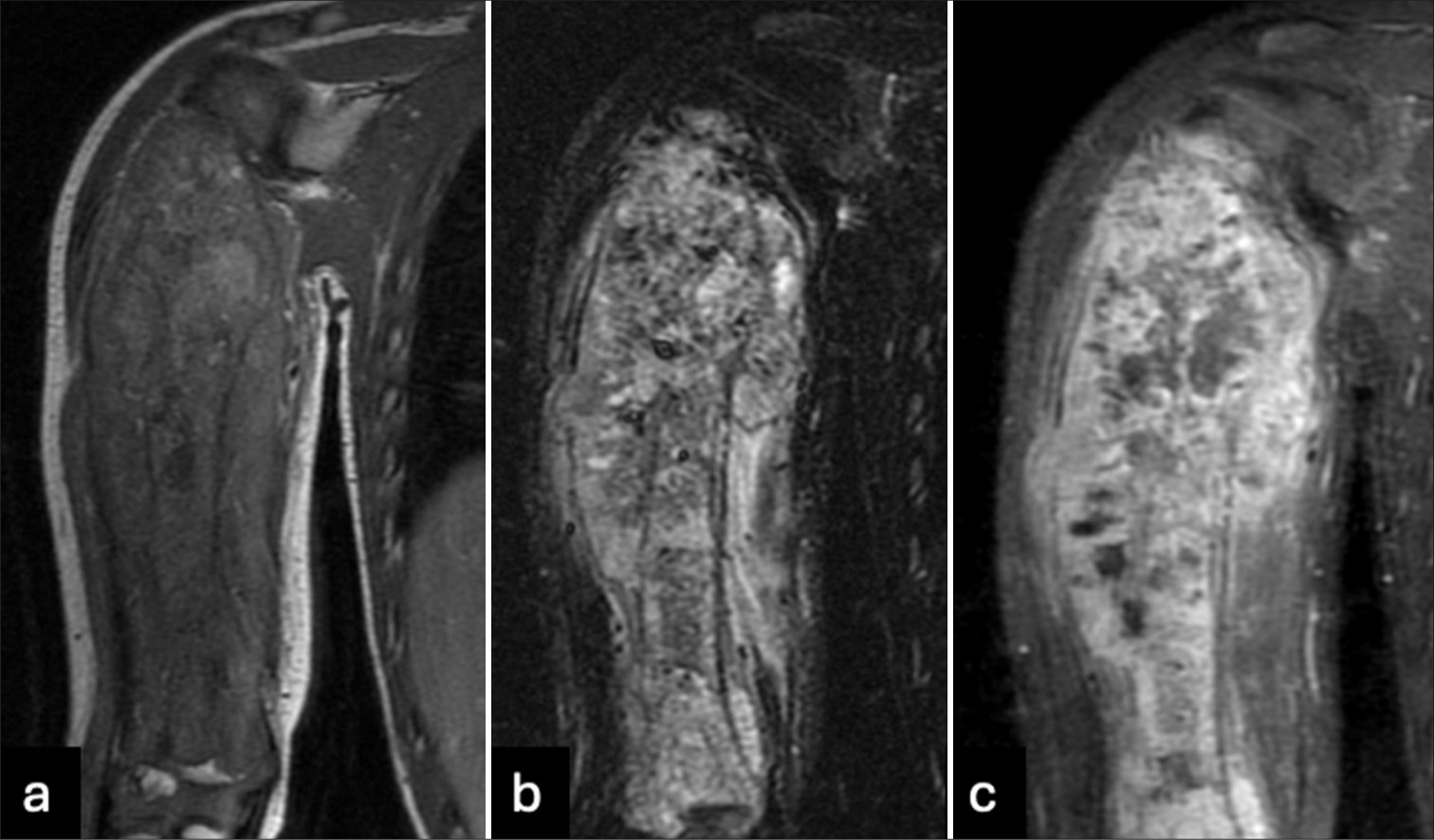
- (a) T1, (b) T2 short tau inversion recovery, and (c) T1 post-contrast images of the right humerus show a diffuse and aggressive infiltrative process involving the length of the humerus with a pathological fracture of the proximal diaphysis. There is an asymmetrically thick circumferential rind of heterogeneously enhancing soft tissue mass. There is transphyseal extension into the humeral head with suspected involvement of the glenohumeral joint.
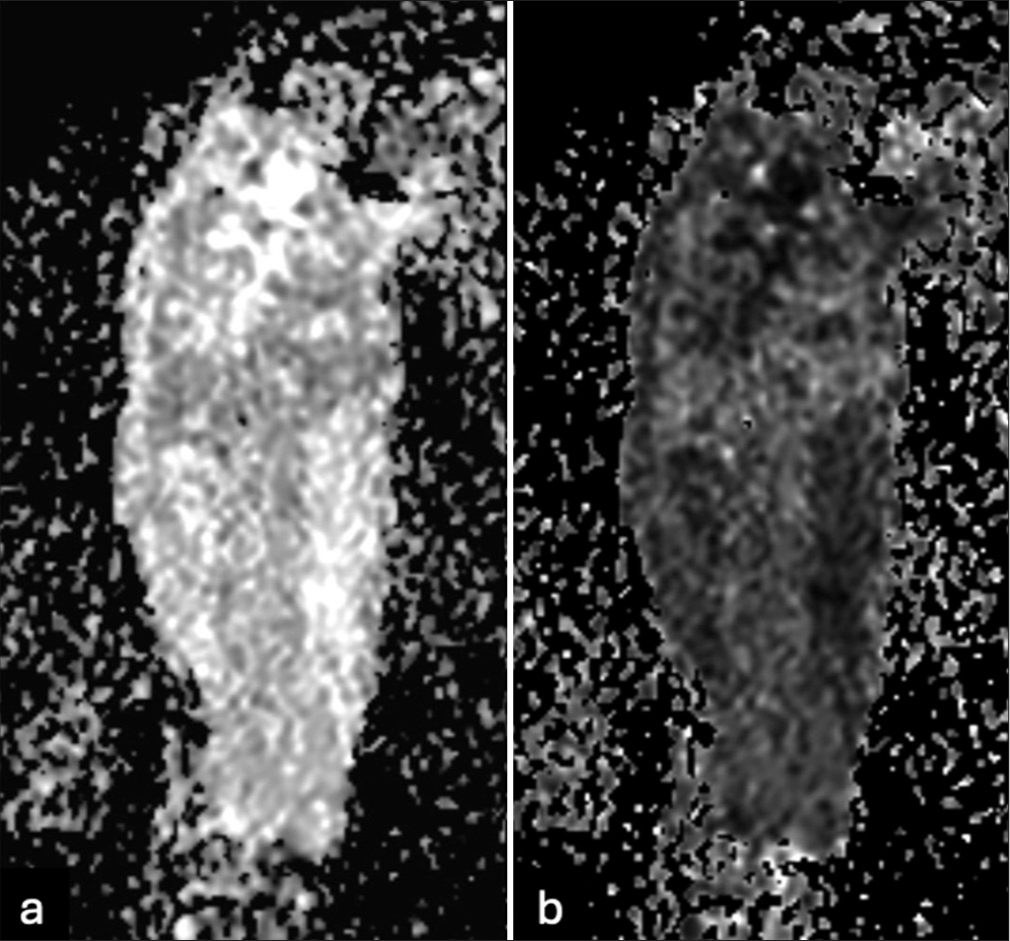
- (a) Diffusion-weighted imaging and (b) apparent diffusion coefficient maps show restricted diffusion throughout the osseous and extraosseous components of the diffusely infiltrative process involving the entire humerus length.
The patient was referred to a pediatric cancer facility for further care. The biopsy results confirmed a diagnosis of OS. Whole body positron emission tomography/computed tomography (PET-CT) [Figure 4] was consistent with a hypermetabolic tumor involving the entire right humerus with surrounding soft tissue. There was no evidence of local or distant hypermetabolic metastatic disease.
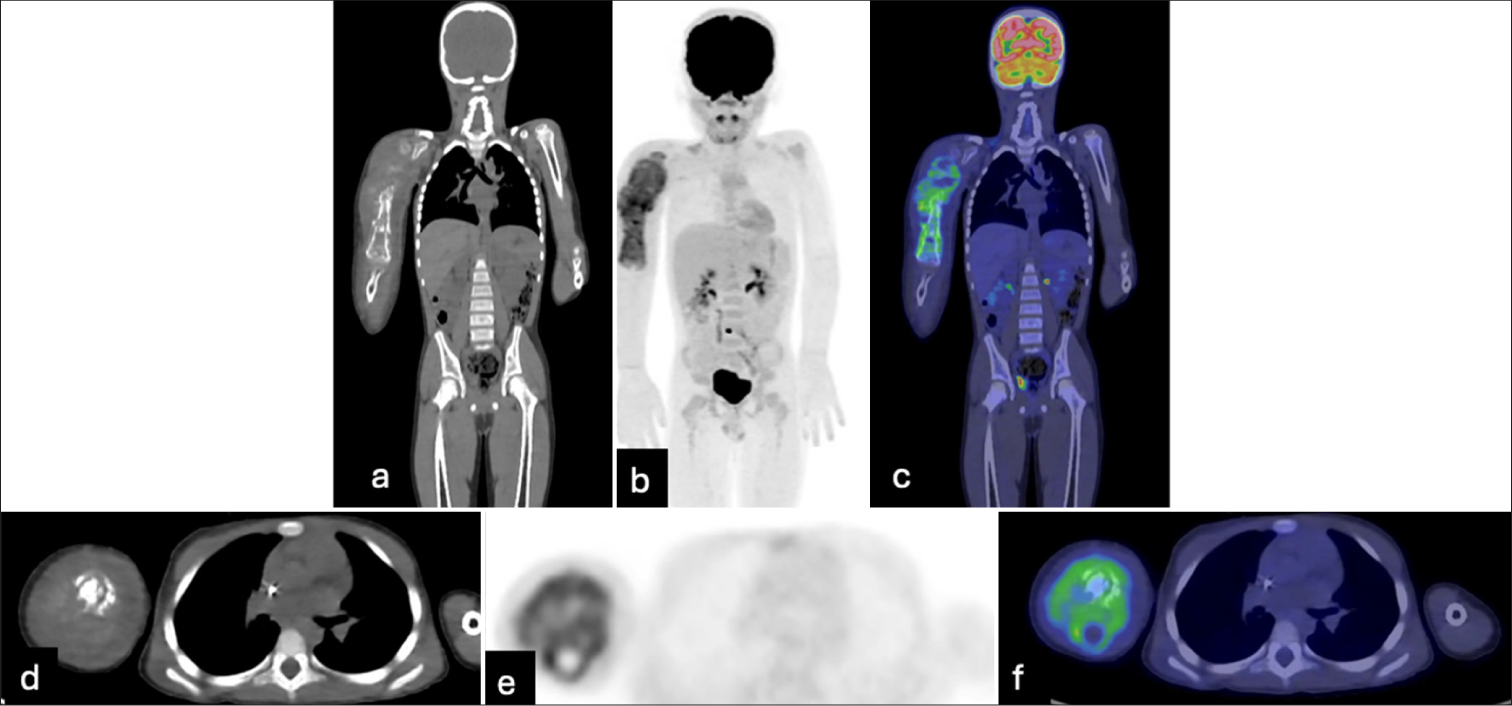
- Whole-body positron emission tomography/computed tomography demonstrates a hypermetabolic tumor involving the entire right humerus with surrounding soft tissue components. There is no evidence of local or distant hypermetabolic metastatic disease.
The proposed treatment plan was a combination of chemotherapeutic agents (cisplatin, doxorubicin, and methotrexate) before surgical resection with limb-sparing procedure or amputation.
The patient had follow-up radiographs of the right humerus [Figure 5] after 2 cycles of chemotherapy, which demonstrated marked increase in thickness of heterogeneous calcification along the length of the humerus with increased periosteal reaction. MRI [Figure 6] showed increased necrosis within the mass and increased size and extent of extraosseous components. He eventually underwent amputation of the right upper extremity with right shoulder disarticulation. The final pathology report confirmed a high-grade conventional OS with 65% necrosis.
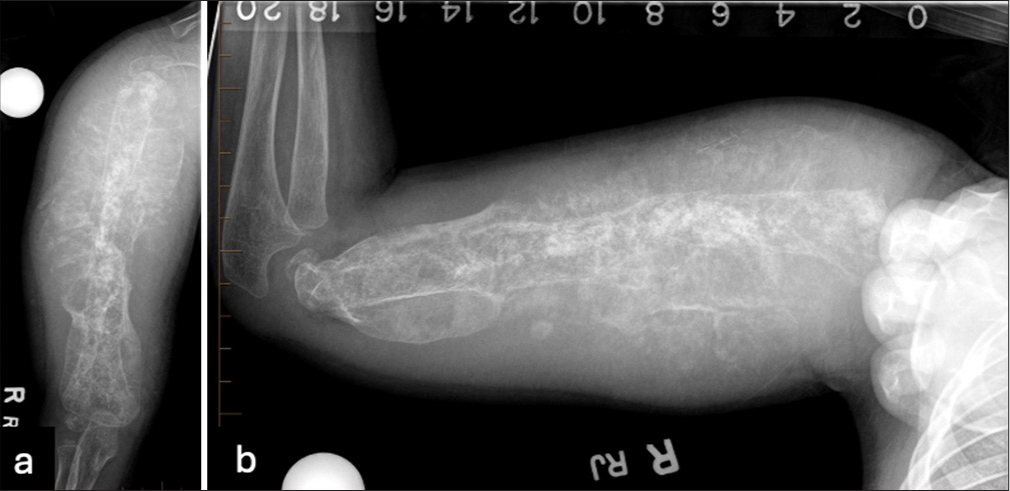
- (a) Antero-posterior and (b) lateral radiographs of the right humerus demonstrate an extensive lytic and sclerotic process involving the entire length of the humerus with a marked increase in thickness of heterogeneous calcification along the length of the humerus. The abnormality seen along the cortex has a more organized morphology, especially distally. The cortical and medullary detail is partially obscured by extensive periosteal new bone formation.

- (a) T1, (b) T2, and (c) T1 post contrast images of the right humerus show a worsened aggressive process involving the entire humerus extending from the proximal to distal epiphyses with an associated pathological fracture of the proximal diaphysis. There is an increased extent of osseous and extraosseous components. There are non-enhancing components suggestive of necrosis, the extent of which has increased compared to the prior magnetic resonance imaging.
DISCUSSION
OS is an aggressive bone tumor known to have a bimodal age of occurrence with characteristic locations and imaging appearance.[1,2] Our patient presented at a much younger age with involvement of the entire length of the humerus from proximal to distal epiphyses, which is quite unusual.
While the appendicular skeleton remains the most frequent location for OS, less common sites include the proximal humerus, scapula, and flat bones of the pelvis. These atypical presentations can mimic other pathologies, posing a diagnostic challenge. Within the appendicular skeleton, the most common site of involvement is the metaphyses of long bones. Involvement of the diaphysis is rare, accounting for <10% of OS cases.[8] Diaphyseal OS has a lower incidence, a higher age of onset, and a larger extent of tumor, with a more osteolytic pattern.[9] Diaphyseal OS can have a variety of histologic patterns including osteoblastic, chondroblastic, telangiectatic, hemangiopericytomatous, small cell, and spindle cell pattern,[10] [Figure 7].
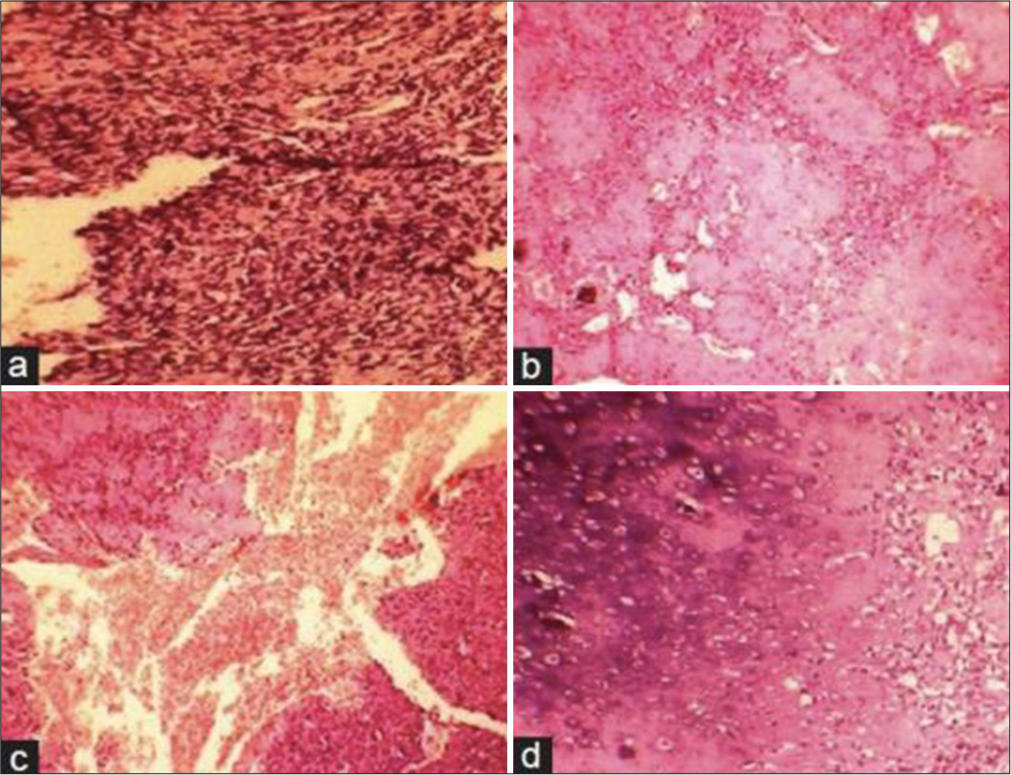
- Photomicrograph showing (a) malignant spindle-shaped cells (b) Osteoid with pleomorphic cells (c) Large areas of hemorrhage with endothelial-lined vascular areas and neoplastic cells (d) Chondroblastic areas (H&E, x100).
The optimal approach to OS diagnosis often involves a combination of imaging modalities.[5] Plain radiographs offer a rapid initial assessment, while CT and MRI provide detailed information for definitive diagnosis and treatment planning. Integration of these modalities allows for a comprehensive evaluation of the tumor and potential metastases.
Plain radiography remains the first-line imaging modality for suspected OS due to its accessibility and cost-effectiveness.[5] Radiographic findings suggestive of OS include lytic or mixed lytic-sclerotic lesions, bone destruction, periosteal reaction, and soft tissue involvement. However, plain radiographs have limitations in terms of soft tissue detail and differentiation from benign mimics.
Computed tomography provides detailed information on bone structure, extent of tumor involvement, and potential cortical breach.[5] They are particularly valuable for pre-surgical planning and evaluation of lung metastases. PET-CT can help identify metabolically active disease and potential distant metastases.
Magnetic resonance imaging depicts soft tissue involvement, marrow infiltration, and relationship with adjacent structures.[5] It is crucial for differentiating OS from other bone lesions and guiding biopsy site selection. According to Nguyen et al.,[5] proximal intramedullary spread of OS into the epiphysis can be present in up to 80% of cases and extension into the glenohumeral joint in 19–24% of cases. Neurovascular involvement is another factor that can be assessed on MRI. It is critical to identify these factors preoperatively as they are associated with a poorer prognosis and directly impact the surgical approach. [5]
It is important to include OS in the differential for an aggressive bone lesion in the pediatric age group irrespective of age, location, and extent of involvement. Early and accurate diagnosis of OS is crucial for optimal patient outcomes, and diagnostic imaging plays an indispensable role in this process.
CONCLUSION
The diagnostic approach for osteosarcoma, an aggressive bone tumor with characteristic imaging appearances, often involves a combination of imaging modalities, including plain radiographs, CT, and MRI for adequate assessment. While osteosarcoma has a predilection for metaphyses of long bones, the presented case with extensive humeral involvement underscores its unusual presentations. The less frequent diaphyseal involvement coupled with diverse histological patterns adds to the diagnostic complexity. Comprehensive imaging is therefore essential for definitive diagnosis, treatment planning, and the evaluation of potential metastases.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship: Nil.
References
- Imaging characteristics of primary osteosarcoma: Nonconventional subtypes. Radiographics. 2010;30:1653-72.
- [CrossRef] [PubMed] [Google Scholar]
- The top 100 most-cited articles on osteosarcoma: A bibliometric analysis. Int J Surg Oncol. 2018;3:e62.
- [CrossRef] [Google Scholar]
- Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National cancer data base report. Clin Orthop Relat Res. 2007;459:40-7.
- [CrossRef] [PubMed] [Google Scholar]
- Osteosarcoma: A review with emphasis on pathogenesis and chemoresistance. Med Res Arch. 2020;8:1-37.
- [CrossRef] [Google Scholar]
- Pediatric osteosarcoma: Correlation of imaging findings with histopathologic features, treatment, and outcome. Radiographics. 2022;42:1196-213.
- [CrossRef] [PubMed] [Google Scholar]
- Osteosarcoma: Radiologic-pathologic correlation and staging. Radiographics. 2017;37:552-70.
- [Google Scholar]
- Pathogenesis and current treatment of osteosarcoma: Perspectives for future therapies. J Clin Med. 2021;10:1182.
- [CrossRef] [PubMed] [Google Scholar]
- Diaphyseal osteosarcomas have distinct clinical features from metaphyseal osteosarcomas. Eur J Surg Oncol. 2014;40:1095-100.
- [CrossRef] [PubMed] [Google Scholar]
- Primary diaphyseal osteosarcoma in long bones: Imaging features and tumor characteristics. Eur J Radiol. 2012;81:3397-403.
- [CrossRef] [PubMed] [Google Scholar]
- Diaphyseal osteosarcoma with varying histomorphologic patterns. Adv Biomed Res. 2014;3:33.
- [CrossRef] [PubMed] [Google Scholar]






