Translate this page into:
Imaging in Gorlin–Goltz Syndrome with Emphasis on Diffusion-Weighted Imaging

*Corresponding author: Saurabh Maheshwari, Department of Radiodiagnosis and Imaging, Armed Forces Medical College, Pune - 411 040, Maharashtra, India. saurabhmhshwr@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Maheshwari S, Dudhal R, Rajesh U, Prateek. Imaging in Gorlin–Goltz Syndrome with Emphasis on Diffusion-Weighted Imaging. Indian J Musculoskelet Radiol 2020;2(2):128-32.
Abstract
Nevoid basal cell carcinoma syndrome is a hereditary condition associated with a wide range of developmental anomalies as well as increased risk of certain neoplasms. It is more commonly known as Gorlin–Goltz syndrome (GGS). We report a case of GGS with classic clinical and imaging findings of multiple odontogenic keratocysts (OKCs) in the jaw, calcification of falx cerebri, and bifid ribs. The findings of restricted diffusion within OKCs on diffusion-weighted imaging are also described.
Keywords
Gorlin–Goltz syndrome
Nevoid basal cell carcinoma syndrome
Nevoid basal cell carcinoma syndrome
Odontogenic keratocyst
Bifid rib
INTRODUCTION
Nevoid basal cell carcinoma (BCC) syndrome is characterized by a classic triad of multiple basic cellular epithelial neoplasms, odontogenic keratocysts (OKCs), and rib anomalies along with numerous other developmental anomalies and a propensity to develop various neoplasms. It is known more commonly as Gorlin–Goltz syndrome (GGS) due to its first detailed description in 1960 by Dr. Robert Gorlin and Robert Goltz.[1] We report the case of a 12-year-old girl with classic features of GGS.
CASE REPORT
A 12-year-old girl presented to the dental department of our tertiary care center in Southern India with a painless, gradually progressive, bony swelling on the right side of the face [Figure 1]. Clinical examination revealed a large swelling over the right half of the face. The surface of the swelling was bony hard. It was non-fluctuant and non-tender. The swelling was causing facial dysmorphism on the right side. A few smaller swellings were also palpable overlying the rest of the mandible. No other abnormality was detected. Developmental and family history did not yield any significant information.

- Frontal photograph of the patient shows presence of swelling on the right side of face with associated facial dysmorphism.
An orthopantomogram and a skull anteroposterior view showed multiple expansile radiolucent cysts within the maxillary and mandibular alveolus [Figure 2]. These cysts showed smooth corticated borders, and some of them were associated with unerupted teeth. In view of radiographic findings and the multiplicity of the lesions in a young patient, a suspicion of multiple OKCs related to GGS was raised. The patient was referred to our department to determine the extent of the lesions and surgical planning.
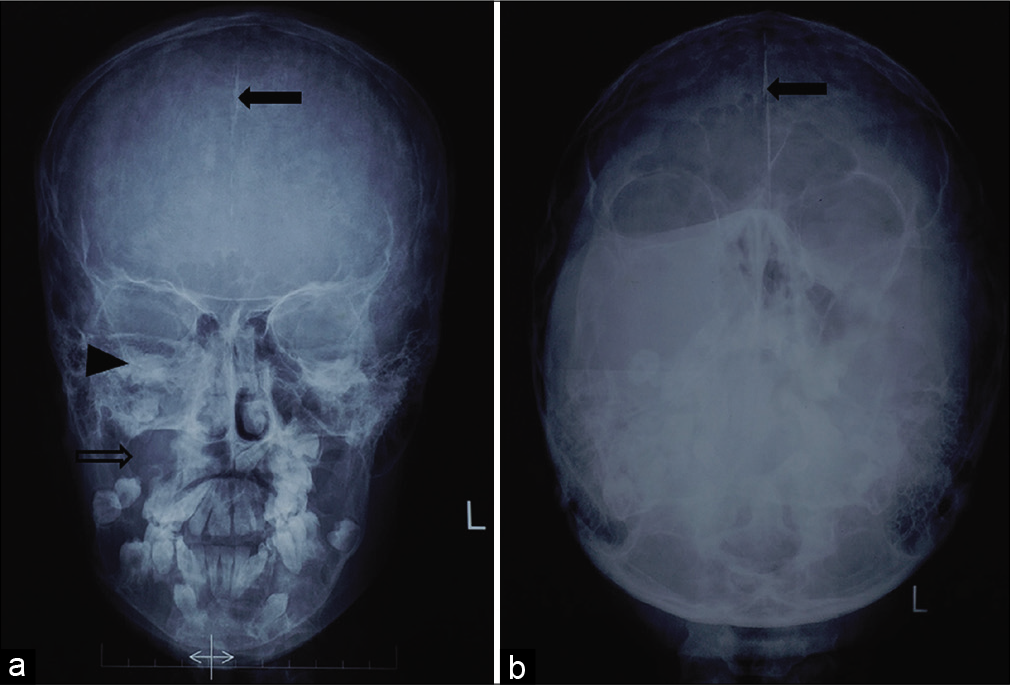
- Radiograph Water’s view of paranasal sinuses (a) and Towne view of skull (b) show the presence of multiple lytic lesions in maxillary and mandibular alveolus with a few of them associated with unerupted teeth. The margins of right maxillary sinus and right orbit are also distorted (black arrowhead). The largest lesion is seen in the right maxillary alveolus (void arrow). Note is also made of increased density in the midline suggestive of calcification of falx cerebri (solid arrows).
With suspicion of GGS, we performed a Water’s view of paranasal sinuses which showed falx cerebri calcification [Figure 2]. A chest radiograph PA view was also done to look for rib anomalies that showed bifid ribs [Figure 3]. These findings confirmed the diagnosis of GGS. Ultrasound of the abdomen and pelvis and radiographic screening of the spine showed no other abnormalities.
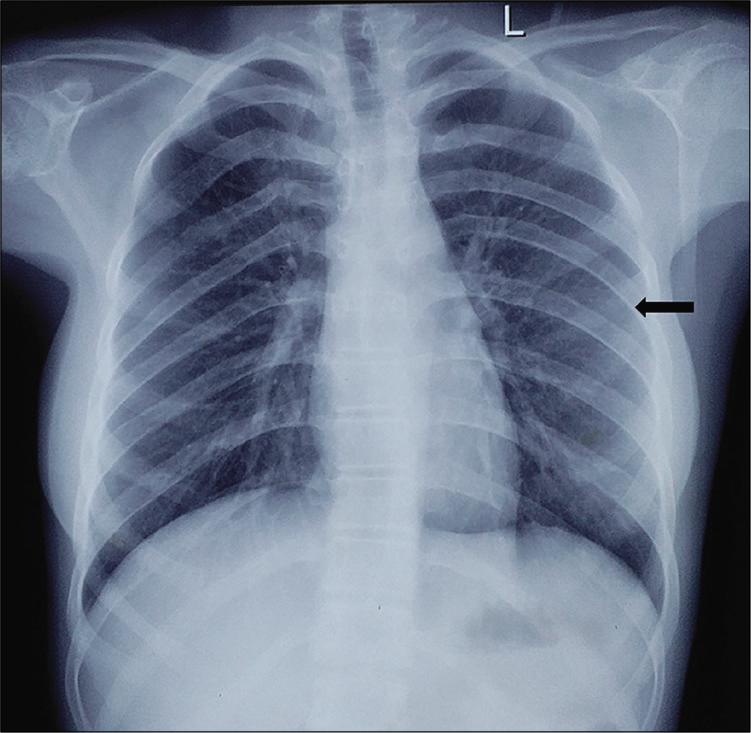
- Chest radiograph posteroanterior view showing left bifid 4th rib (black arrow).
Targeted clinical evaluation (including dermatology and neurology consultation) for other congenital anomalies associated with GGS was negative. Routine hematological and biochemical investigations were within the normal physiological range.
A contrast-enhanced computed tomography (CT) scan of the face was performed on a 16-slice CT scanner for delineating the extent of the lesion and for pre-operative planning. It showed multiple unilocular expansile cystic lesions filled with fluid attenuation contents in maxillary and mandibular alveolus with an appearance similar to radiographs [Figure 4]. No septae, differential attenuation contents, solid component, or post-contrast enhancement were seen within these lesions. The largest of these lesions were seen in the right half of maxillary alveolus causing complete effacement of the right maxillary sinus and causing scalloping and pressure deossification of the maxillary alveolus. Furthermore, bilamellar calcification of the falx cerebri and tentorium was confirmed [Figure 4]. There was no evidence of any intracranial neoplasms.
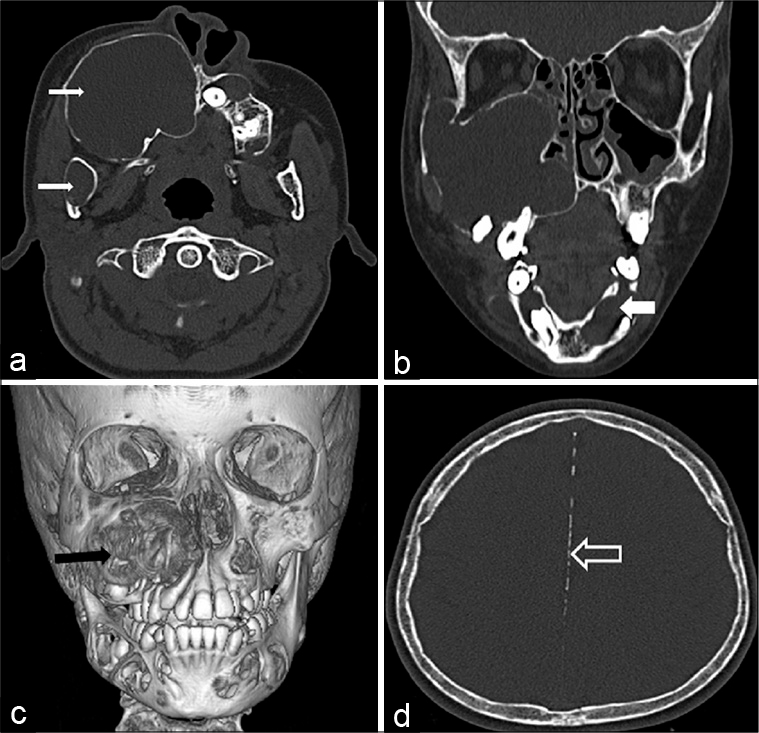
- Computed tomography images in axial plane (a) and coronal reformat (b) in bone window showing multiple expansile lytic lesions in maxillary and mandibular alveolus (white solid arrows). A three-dimensional volume rendered image shows the largest lesion in the right maxillary alveolus (black arrow) along with multiple other lytic lesions. Axial image of brain in bone window (d) shows linear calcification of falx cerebri (void arrow).
Corroborative magnetic resonance imaging (MRI) of the face was performed with the acquisition of multiplanar T1-weighted images (T1WI), T2-weighted images (T2WI), gradient-echo images, and diffusion-weighted images (DWI) followed by post-gadolinium T1-weighted fat-saturated images. The lesions in jaw appeared homogeneously hypointense on T1WI and hyperintense on T2WI. No susceptibility artifacts were seen within these lesions. These lesions demonstrated predominantly peripheral areas of restriction of diffusion within [Figure 5]. The apparent diffusion coefficient (ADC) value in areas of restriction of diffusion was 0.73 × 10−3 mm2/s.
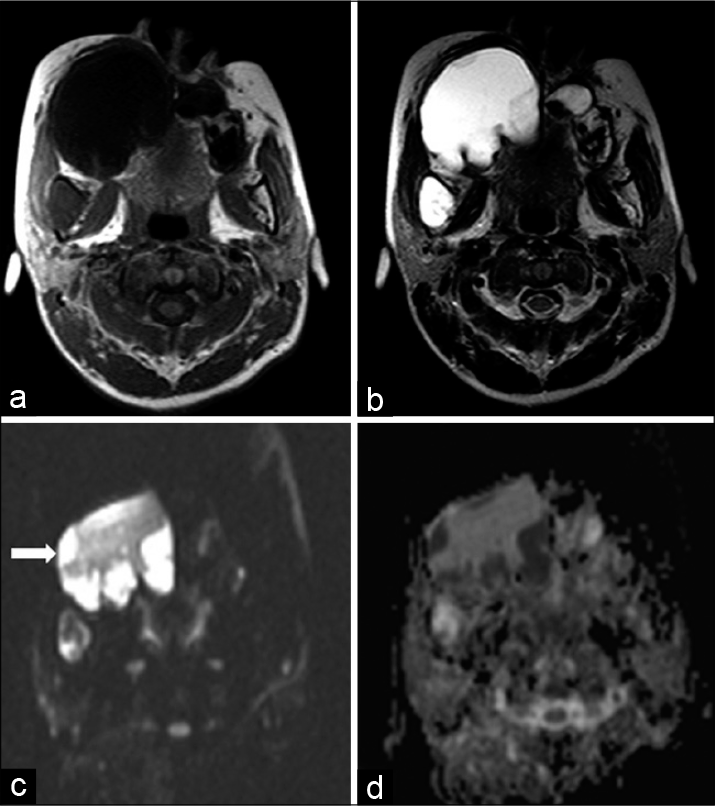
- Magnetic resonance imaging show the lesions in the jaw to be hypointense on T1 weighted image (a) and hyperintense on T2-weighted image (b) in the axial plane. The lesion in the right maxillary alveolus shows predominantly peripheral restriction of diffusion on axial diffusion-weighted images (white arrow in figure c) and appears correspondingly dark on apparent diffusion coefficient map (d).
Our patient fulfilled the diagnostic criteria for GGS. She was managed with resection of the largest lesion in the right maxillary alveolus. Post-operative recovery was uneventful. Histopathological examination confirmed the diagnosis of OKC. The patient and her parents were counseled about the other possible symptoms associated with the syndrome and the risk of other neoplastic conditions. The patient is on regular follow-up without any significant post-operative complications.
DISCUSSION
Gorlin and Goltz were the first to describe NBCSS as a distinct syndrome in 1960.[1] However, this condition has been described in the literature as early as 1894.[2] Its diagnostic criteria were first defined in 1993[3] with the latest modification in 1997.[4] It is listed in Figure 6. The diagnosis requires the fulfillment of two major or one major and two minor criteria. Our patient fulfilled three of the major criteria for this syndrome.
![Diagnostic critria for Gorlin–Goltz syndrome.[4]](/content/107/2020/2/2/img/IJMSR-2-128-g006.png)
- Diagnostic critria for Gorlin–Goltz syndrome.[4]
It is an infrequent autosomal dominant inherited condition with a prevalence varying from 1/57,000 to 1/256,000 with a male/female ratio of 1:1.[5] It shows high penetrance with variable expressivity. It is caused by a mutation in the PTCH 1 gene at chromosome 9q22.3-q31, and in up to 60% of cases, there is no family history of this condition.[5] PTCH1 is a tumor suppresser gene. It plays an important part in cell cycle and embryonal structuring. There is a truncation of its C-terminal after mutation, leading to loss of its suppresser function.
The classic clinical manifestation associated with GGS is BCC which may be seen as early as 2 years of age. However, more commonly, the patients present with multiple OKCs in the 2nd to 3rd decade.
The detection of any of the major criteria, even incidentally, should prompt a search for the detection of other criteria. The radiologist, hence, plays a key role in the diagnosis of GGS. Such patients benefit from follow-up for early detection of skin malignancies as up to 80% of the white population and 38% of blacks develop BCCs.[6]
The common imaging manifestations of this entity include lucent jaw lesions (OKCs) in 90%; calcification of falx cerebri (70–85%), tentorium cerebelli (20–40%) and diaphragma sellae (60–80%), macrocephaly (70%), and rib anomalies in the form of bifid, fused, or splayed ribs (30–60%). Other uncommon imaging findings are cleft lip and cleft palate (5%) and vertebral anomalies (craniovertebral junction malformations and cervical and upper thoracic vertebral fusion in 10–40%). Multiple other neoplastic conditions are also seen at a lower frequency, including medulloblastoma (1–2%), meningioma (3–5%), and ovarian fibroma (25–50% of all female patients).
On DWI, our case showed a restriction of diffusion with low ADC values in OKCs. This finding finds little mention in the literature and is worth reiterating. Low ADC values in OKCs were first described by Sumi et al. as a means to differentiate OKCs from ameloblastomas with a cut off ADC value of 2.0 × 10−3 mm2/s.[7] They proposed that the presence of desquamated keratin leads to an increase in the viscosity of the cyst fluid, leading to restricted diffusion. This distinction is useful for surgical planning as ameloblastomas need surgical resection, whereas many OKCs can be treated with enucleation alone. Similar findings were also reported in two other studies.[8,9]
Although multiple OKCs are almost always seen in GGS, a few other conditions are associated with multiple OKCs.
These include Ehlers–Danlos syndrome, Noonan syndrome, and oral-facial-digital syndrome.[10] In some rare cases, nonsyndromic multiple OKCs have also been described.
A multidisciplinary team approach is essential for the successful management of these patients. A dermatological consult with regular surveillance is needed for early identification and treatment of BCCs. Ultraviolet exposure should be avoided in these patients. Jaw keratocysts often need surgical treatment and tend to recur in up to 60% of patients.
CONCLUSION
This case highlights the importance of awareness and high index of suspicion for this rare syndrome in young individuals, especially those without skin lesions. The presence of a major criterion, even if detected incidentally, should prompt the search for other associations. This, in turn, can lead to early detection of malignancies, thus reducing morbidity and mortality. Our case illustrates the classic imaging findings of this condition. The MRI findings of OKCs on DWI are also stressed on, which find the relatively little description in the literature.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908-12.
- [CrossRef] [PubMed] [Google Scholar]
- To teach from the swollen skin. Arch Dermatol Syphil (Berl). 1894;28:162-222.
- [CrossRef] [Google Scholar]
- Complications of the naevoid basal cell carcinoma syndrome: Results of a population based study. J Med Genet. 1993;30:460-4.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- [CrossRef] [Google Scholar]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32.
- [CrossRef] [PubMed] [Google Scholar]
- Nevoid basal cell carcinoma syndrome: A review of the literature. Int J Oral Maxillofac Surg. 2004;33:117-24.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted MR imaging of ameloblastomas and keratocystic odontogenic tumors: Differentiation by apparent diffusion coefficients of cystic lesions. AJNR Am J Neuroradiol. 2008;29:1897-901.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted imaging in the evaluation of odontogenic cysts and tumours. Br J Radiol. 2012;85:e864-70.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted MR imaging of unicystic odontogenic tumors for differentiation of unicystic ameloblastomas from keratocystic odontogenic tumors. Korean J Radiol. 2018;19:79-84.
- [CrossRef] [PubMed] [Google Scholar]
- Odontogenic keratocyst: Imaging features of a benign lesion with an aggressive behaviour. Insights Imaging. 2018;9:883-97.
- [CrossRef] [PubMed] [Google Scholar]







