Translate this page into:
Imaging of a rare sarcoma: A case series of dermatofibrosarcoma protuberans treated in a Tertiary Care Center

*Corresponding author: Sujay Kumar Das, Department of Radiodiagnosis, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. dsujayk22@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Das SK, Meena L, Goel S, Phulware RH, Syed A. Imaging of a rare sarcoma: A case series of dermatofibrosarcoma protuberans treated in a Tertiary Care Center. Indian J Musculoskelet Radiol 2022;4:31-7.
Abstract
Dermatofibrosarcoma protuberans (DFSP) is a rare, low-to-intermediate grade locally aggressive sarcoma. It typically arises in the dermis and infiltrates subcutaneous tissue, predominantly of the trunk and extremities. It has a propensity to recur locally. There are infrequent incidences of distant metastases. The purpose of this study was to know about different imaging modalities in the assessment and management of DFSP. A retrospective study was done on a series of four patients who presented to our institute with complaints of progressive swellings in different regions of the body. A clinical diagnosis of soft-tissue sarcoma was made. They underwent radiography/ ultrasonography/computed tomography/magnetic resonance imaging in our department followed by excisional biopsy. There were certain imaging features suggestive of DFSP. On excisional biopsy, all were proven to be DFSP. There was surgical resection in all the reported cases. After 6 months of follow-up, there was local recurrence in two of the cases. We came to the conclusion that, due to the superficial appearance of DFSP, common lesions are initially thought of which leads to misdiagnosis. Imaging techniques are not always utilized and there is a delay in their proper management. Knowledge of the variable imaging appearances of DFSP may help in the diagnosis of difficult and atypical case scenarios and help in proper early management.
Keywords
Dermatofibrosarcoma protuberans
Computed tomography
Magnetic resonance imaging
Ultrasonography
INTRODUCTION
Dermatofibrosarcoma protuberans (DFSP) is a relatively rare entity among spindle cell tumors. Darier and Ferrand were the first to describe it in 1924.[1] The term “dermatofibrosarcoma protuberans” was given by Hoffman in 1925.[2] This lesion commonly arises in the dermis and tends to spread into the subcutaneous tissues and muscles.[3] The trunk is the most common location of DFSP, with nearly half of all cases found in the trunk. The next most common locations are the extremities followed by the head and neck. However, it can be found anywhere in the body.[4] DFSP is reported to have a high rate of local recurrence and a low tendency to metastasize.[5] The imaging features of DFSP are not much specific but may help with its assessment and diagnosis. A definitive diagnosis can be obtained only by histopathological and immunohistochemical analysis.[6] A clear margin of resection is required during surgery because of the high incidence of recurrence.[7]
MATERIAL AND METHODS
A retrospective study was done on a series of four patients who presented to our institute with complaints of progressive swellings in different regions of the body. A clinical diagnosis of soft-tissue sarcoma (STS) was made.
The patients were referred to the department of radiodiagnosis and imaging for the extent of STS. All of them underwent magnetic resonance imaging (MRI) in either 1.5 T (Siemens Magnetom Aera) or 3 T (GE Discovery MR750w) units. Ultrasonography (USG) (both grayscale and color Doppler, using a high-frequency linear probe: L 3-11 appleprobe in Esaote’s MyLab™9 ultrasound machine) was done in two patients. Computed tomography (CT) scan using a 128 slices scanner (SOMATOM Definition dual-source) was done on one patient to rule out any metastatic lesion, though rare. X-ray of the forearm in one patient was done to check for any bony involvement.
An excisional biopsy was done in all these cases. Histopathological examination was suggestive of DFSP with areas of sarcomatous differentiation/transformation.
RESULTS
Among the four patients in our case series, two were men and two were women. The age range was 33–52 years. The clinical details and imaging findings are described in detail, as shown in [Table 1].
| Case No. | Age (years)/gender | Clinical details | Magnetic resonance imaging findings | Other imaging findings |
|---|---|---|---|---|
| 1 | 52/M | Swelling in the upper back for ~4 months. Mild pain. Hypertensive, occasional smoker. Insignificant family history | A heterogeneously T1 hypointense well-circumscribed oval lesion was seen at the D6 vertebral body level in the subcutaneous plane overlying right trapezius muscle. It showed a hyperintense signal on the T2FS image with heterogeneous post-contrast enhancement, patchy diffusion restriction, and corresponding low ADC values | On ultrasonography: Subcutaneous iso to a hypoechoic lesion On computed tomography: A nodular, well-circumscribed, soft-tissue attenuation mass was seen epicenter in subcutaneous fat of the upper thoracic wall, showing post-contrast enhancement. No evidence of calcification/perineural spread/osseous destruction/regional lymph node enlargement/distant metastasis seen |
| 2 | 41/F | Swelling in the lower back for ~6 months. Known diabetic on medication. Insignificant family history | A well-defined lobulated T2 hyperintense lesion in subcutaneous plane over right lumbar region showing heterogeneous post-gadolinium enhancement with multiple non-enhancing areas within; fat planes with underlying paraspinal muscles maintained; showing patchy areas of diffusion restriction with corresponding low ADC values |
- |
| 3 | 35/M | Swelling over the lateral aspect of right upper thigh for ~6.5 months. Occasional smoker, otherwise healthy. Pain in the right thigh for the past 2 months | A lesion within the right adductor magnus muscle, with central T1/T2 iso to hypointense and peripheral T2 hyperintense signal. Peripheral post-gadolinium enhancement; patchy areas of diffusion restriction seen within | - |
| 4 | 33/ F | Swelling in the right forearm, mild pain. Ulceroproliferative mass in the posterior aspect of the forearm just below the elbow. No comorbidities; insignificant family history | An exophytic mass lesion seen along posterolateral aspect of the left arm in the subcutaneous plane showing T1 hypointense/T2 hyperintense signal with heterogeneous post-gadolinium enhancement. Medially it is abutting the underlying muscles with maintained fat planes with the muscles on extensor compartment. The lesion shows diffusion restriction with corresponding low ADC values | Radiographs: AP and lateral views showed two exophytic radiopaque lesions along the posterolateral aspect of the left forearm in the subcutaneous plane On ultrasonography: On grayscale ultrasound, a well-defined predominantly hypoechoic mass was seen in the subcutaneous plane with increased vascularity on color Doppler |
The first case [Figure 1] was of a 52-year-old male who presented to the hospital with the complaints of mildly painful swelling in the upper back for about 4 months. He was mild hypertensive and an occasional smoker. On USG, a subcutaneous iso- to hypoechoic lesion was seen without significant vascularity on color Doppler. On contrast-enhanced CT, a nodular, well-circumscribed, and soft-tissue attenuation mass was seen epicenter in subcutaneous fat of the upper thoracic wall, showing post-contrast enhancement. On MRI, a heterogeneously T1 hypointense well-circumscribed oval lesion was seen at the D6 vertebral body level in the subcutaneous plane overlying the right trapezius muscle. It showed a hyperintense signal on the T2FS image with heterogeneous post-contrast enhancement and patchy diffusion restriction and corresponding low ADC values. An excisional biopsy was done, suggestive of DFSP with areas of sarcomatous differentiation. Later complete resection of the tumor was done. On 6 months of follow-up, no recurrence was seen.

- A 52-year-old man with swelling in the upper back. Ultrasonography (USG), contrast-enhanced computed tomography (CECT), and magnetic resonance imaging of the lesion. The lesion is marked with arrows and stars. (a) Axial grayscale USG shows iso- to hypoechoic (relative to adjacent muscle) lesion. (b) Color Doppler image shows no significant internal vascularity. (c) Axial NCCT image shows: A nodular, well-circumscribed, soft-tissue attenuation mass, epicenter in subcutaneous fat of upper thoracic wall on the right paramedian location. The mass stretches the overlying skin, causing a bulge over the back. (d) Axial CECT image showing post-contrast enhancement of the marked lesion. The lesion is superficial to the right trapezius muscles with infiltration of the intervening fat plane. (e) Axial T1W image shows A heterogeneously hypointense well-circumscribed oval lesion in the upper back. (f) Axial T1W post-contrast image showing heterogeneous post-contrast enhancement. (g) Axial post-contrast T1WI shows: Thickening and post-contrast enhancement of underlying fascia of the right trapezius muscle (marked with arrow), post-contrast enhancement in posterolateral fibers of the right trapezius with reduced muscle bulk. (h) Axial T2WI shows a heterogeneously hyperintense signal in the arrow-marked lesion. (i) Axial T2FS image shows hyperintense signal. (j) Axial DWI shows: Patchy diffusion restriction with corresponding low apparent diffusion coefficient (ADC) values (k).
The second case [Figure 2] was of a 41-year-old female who presented to the hospital with complaints of swelling in the lower back on the right side of the midline for about 6 months. She was a known diabetic on medication. On MRI, a well-defined lobulated T2 hyperintense lesion was seen in the subcutaneous plane over the right lumbar region showing heterogeneous post-gadolinium enhancement with multiple non-enhancing areas within; fat planes with underlying paraspinal muscles were maintained. The lesion was showing diffusion restriction with corresponding low ADC values. The lesion was excised, keeping a clear margin and the biopsy was suggestive of DFSP. On 6 months of follow-up, no recurrence was seen.
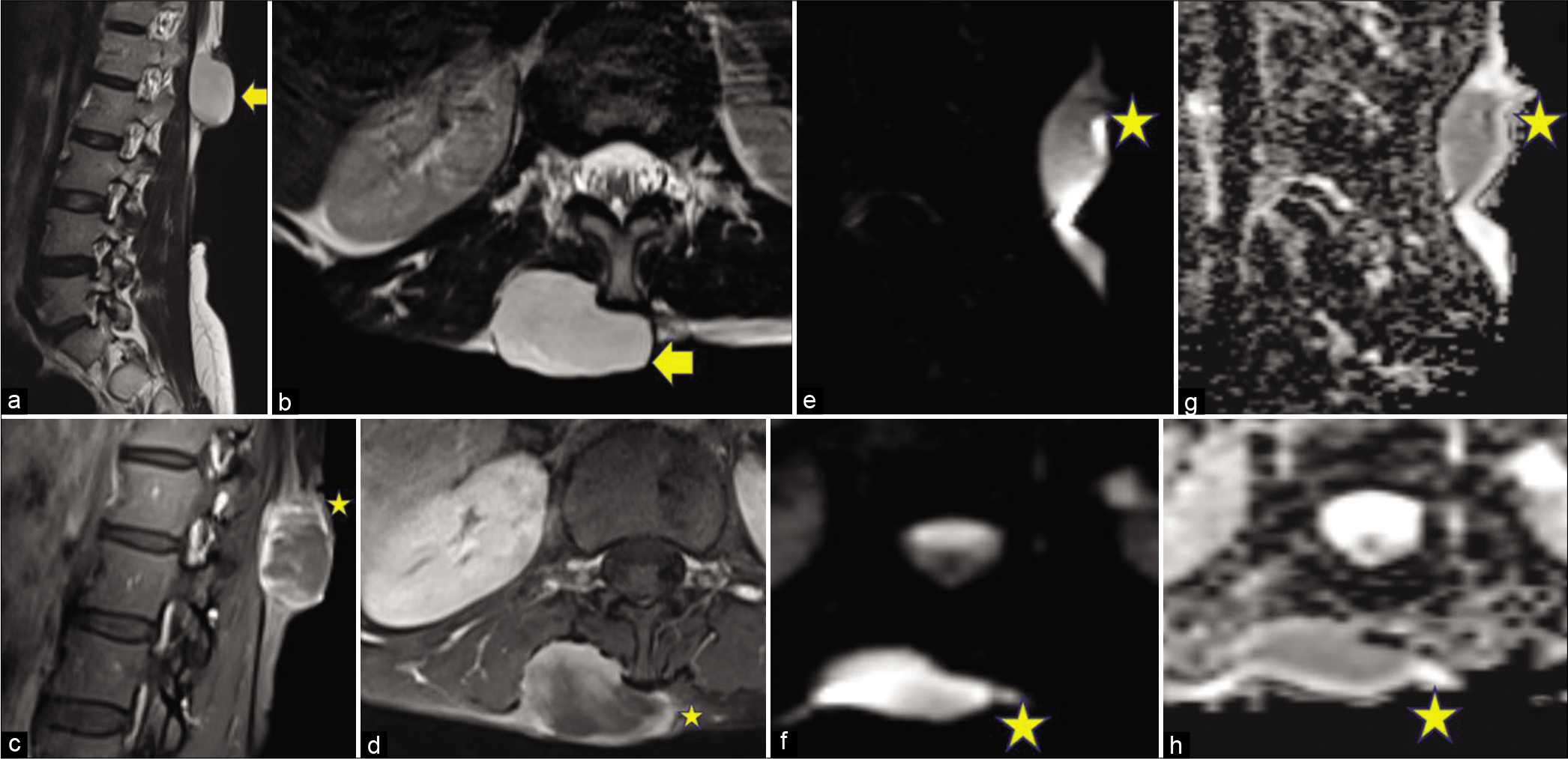
- A 41-year-old female with swelling in the lower back. Magnetic resonance imaging of the lesion. The lesion is marked with arrows and stars. (a) Sagittal T2WI and (b) Axial T2WI show: A well-defined lobulated T2 hyperintense lesion in a subcutaneous plane over the right lumbar region posteriorly extending from the L1-L2 level in the left paravertebral region. (c) Sagittal and (d) axial post-contrast T1WI show: Heterogeneous enhancement of the lesion with multiple non-enhancing areas within. Fat planes with underlying paraspinal muscles are maintained. (e) Sagittal and (f) axial sections, DWI showing: Diffusion restriction in the lesion. (g and h) Sagittal and axial images show: Corresponding low ADC values.
In the third case [Figure 3], a 35-year-old male presented to the hospital with a swelling over the lateral aspect of the right upper thigh for about 6½ months. He was a farmer by occupation and occasional smoker and was otherwise healthy. Previously, he had visited a local hospital for the same complaint and was taking Ayurvedic medication. The swelling was not subsiding and he had pain in the right thigh for the past 2 months. On MRI, a lesion was seen within the right adductor magnus muscle, with central T1/T2 iso- to hypointense and peripheral T2 hyperintense signal. Peripheral post-gadolinium enhancement was seen with patchy areas of diffusion restriction within. A similar subcutaneous lesion in the lateral aspect of the right thigh was also seen, which was likely the primary lesion to the intramuscular lesion. Excisional biopsy confirmed the lesion to be DFSP with areas of sarcomatous differentiation. After 6 months of follow-up, unfortunately, there was a recurrence seen in the subcutaneous location. He underwent complete excision later.
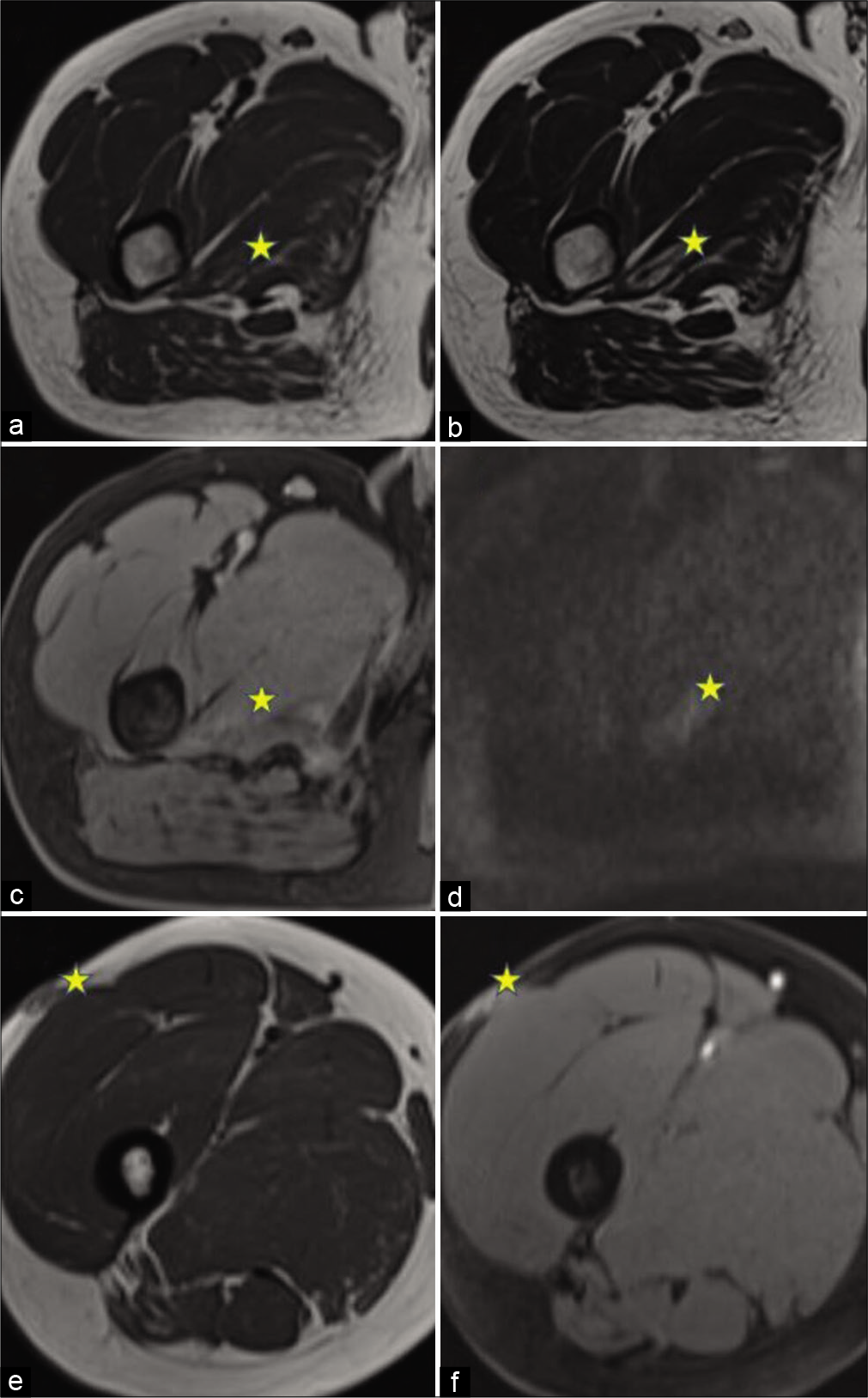
- A 35-year-old man with swelling in the right thigh. magnetic resonance imaging of the lesion. The lesion is marked with stars. (a) Axial T1WI shows a lesion within the right adductor magnus muscle, with central iso- to hypointense and peripheral hyperintense signal. (b) Axial T2WI shows a hyperintense signal in the lesion with the central hypointense signal. (c) Axial T1W post-contrast image shows Mild peripheral enhancement. (d) Axial DWI, showing: Patchy areas of diffusion restriction. (e) Axial T1WI showing the primary subcutaneous lesion with heterogeneous post-gadolinium enhancement (f).
In the fourth case [Figure 4], a 33-year-old female presented to the hospital with a swelling in the right forearm for about 3 months with mild pain over the swelling. On clinical inspection, an ulceroproliferative mass was seen in the posterior aspect of the forearm just below the elbow. She had no comorbidities or significant family history. Radiographs, anterior-posterior, and lateral views of the forearm showed two exophytic radiopaque lesions along the posterolateral aspect of the left forearm in the subcutaneous plane. On USG, a well-defined predominantly hypoechoic mass was seen in the subcutaneous plane with increased vascularity on color Doppler. On MRI, an exophytic mass lesion was seen along posterolateral aspect of the left arm in the subcutaneous plane showing T1 hypointense/T2 hyperintense signal with heterogeneous post-gadolinium enhancement. Medially, it was abutting the underlying muscles with maintained fat planes with the muscles on the extensor compartment. The lesion showed diffusion restriction with corresponding low ADC values. The mass was excised. Biopsy from the mass was suggestive of DFSP with areas of sarcomatous differentiation/ transformation. After 6 months of follow-up, there was a local recurrence and the lesion had to be excised again.
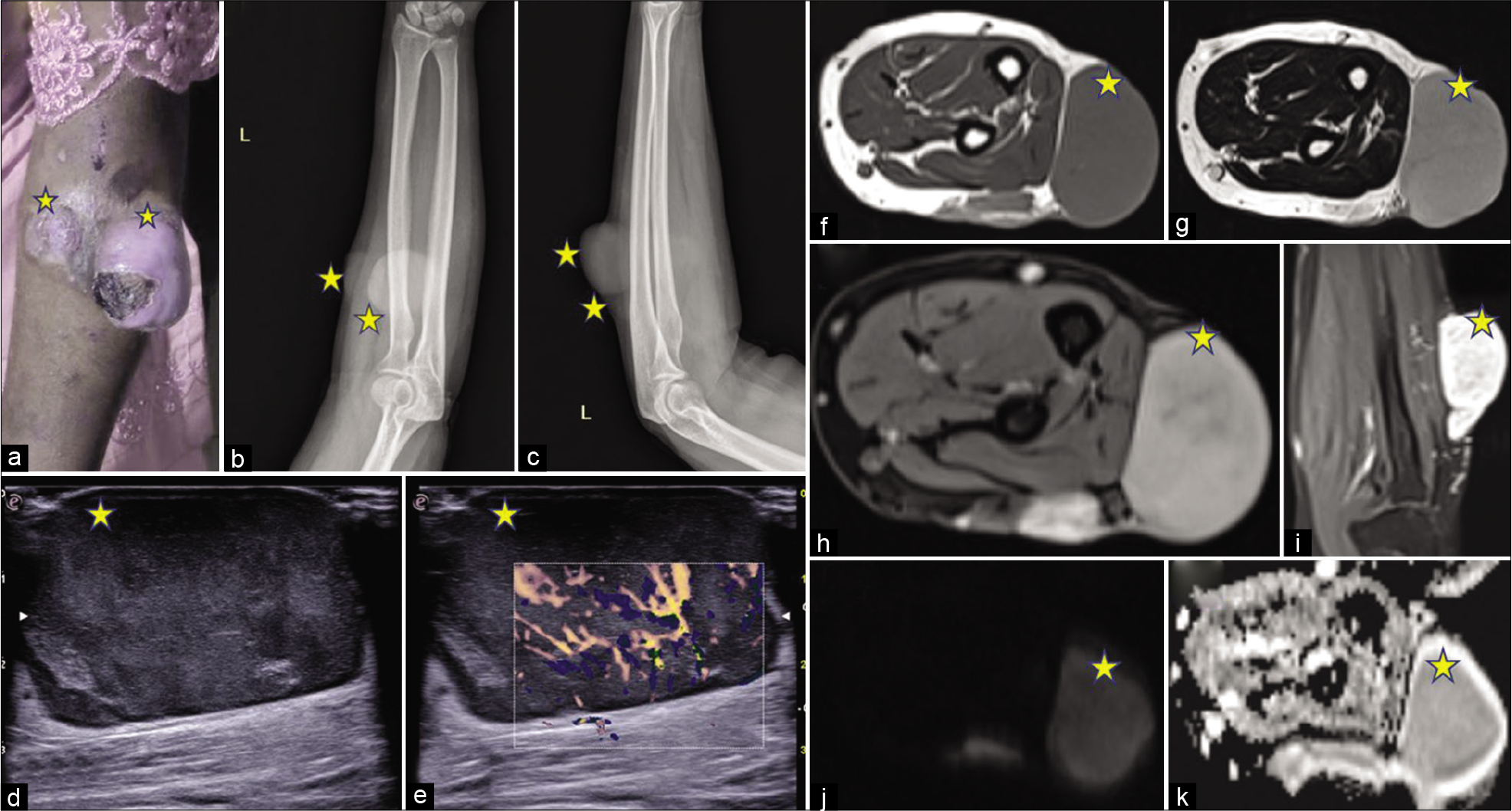
- A 33-year-old female with swelling in the right forearm. Gross appearance, radiograph, ultrasonography, and magnetic resonance imaging of the lesion. Lesions are marked with stars. (a) On clinical inspection: An ulceroproliferative mass is seen in the posterior aspect of the forearm just below the elbow. (b) Radiograph of the left forearm, AP view shows Two exophytic soft-tissue density lesions (marked with stars) along the posterolateral aspect of the left forearm in the subcutaneous plane. (c) Radiograph of the left forearm, lateral view showing the soft-tissue density lesions (marked with stars). (d) A grayscale ultrasound image shows A well-defined predominantly hypoechoic mass in the subcutaneous plane. (e) Color Doppler ultrasonography shows Increased vascularity within the lesion. (f) T1W image, (g) T2WI showing a T1 hypointense and T2 hyperintense exophytic mass lesion along posterolateral aspect of the left arm in the subcutaneous plane with heterogeneous enhancement on a post-gadolinium image, (h) axial and (i) coronal post-gadolinium image. Medially, it is abutting the underlying muscles with maintained fat planes with the muscles on the extensor compartment. (j) DWI shows: Diffusion restriction with corresponding low ADC values (k).
The common imaging features in the cases of this case series were as follows: Hypointensity on T1-weighted image (T1WI); hyperintensity on T2-weighted image (T2WI); heterogeneous post-gadolinium enhancement; and patchy areas of diffusion restriction on MRI. On USG, predominantly hypoechoic lesion was found with increased vascularity.
The history of hypertension and smoking did not affect any imaging features.
On histopathological examination from a lesion in the first case [Figure 5], sections examined show a tumor exhibiting hypercellular areas alternating with hypocellular areas. Tumor cells are arranged in fascicles showing whorling patterns, intersecting fascicles, and focal storiform patterns. Cells are oval to spindle-shaped with elongated nuclei vesicular chromatin conspicuous nucleoli and mild pleomorphism. Sarcomatous components showed marked nuclear pleomorphism, hyperchromatic nuclei, and prominent nucleoli with mitosis (14–16/10 HPF). There was no evidence of necrosis. No overlying skin was seen in sections examined. All margins of the specimen were involved by the tumor. Epidermis was unremarkable with tumor extension in the dermis and subcutaneous fat. On immunohistochemistry, DFSP component spindle cells were diffusely CD34 positive while the sarcomatous component was negative. On further immunohistochemistry, these tumor cells were immunonegative for desmin, TLE-1, S-100, CD99, podoplanin, Stat-6, FLI-1, Bcl-2, and CD117.
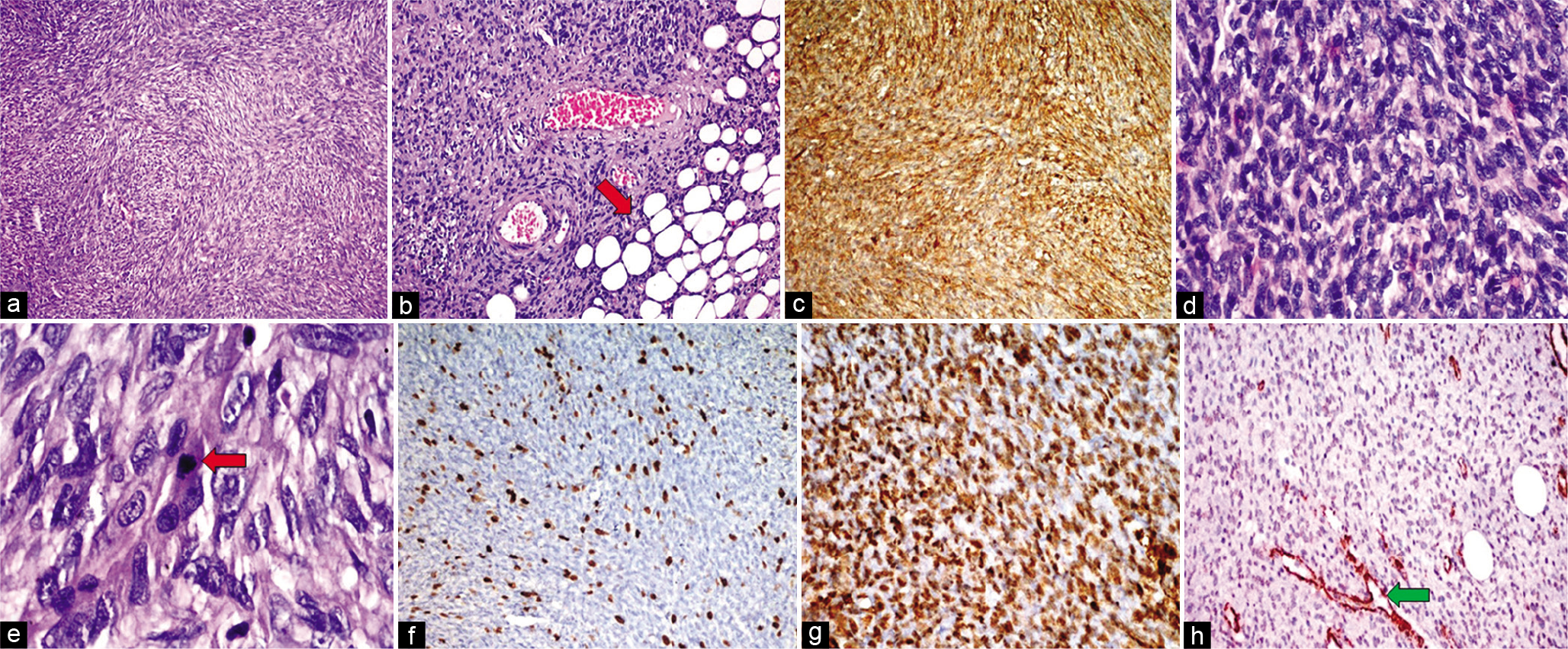
- HPE from lesion in the upper back of Case 1. Hematoxylin and eosin (H&E) stained sections demonstrate a proliferation of spindle-shaped cells with elongated nuclei and mild pleomorphism, arranged in a storiform to the whorled pattern (a ×100). This lesion infiltrates the underlying subcutaneous fat (red arrow), producing a honeycomb-like design (b ×200). Immunohistochemistry (IHC) shows strong and diffuse positivity for CD34 indicating spindle cell proliferation (c). The areas of the sarcomatous component showed marked nuclear pleomorphism (H&E; d ×400), and higher magnification showed hyperchromatic nuclei with atypical mitosis (red arrow) (H&E; e ×1000). The index of ki-67 proliferative activity was approximately 10–12% positive (f). IHC in sarcomatous areas showed diffuse immunopositivity for smooth muscle actin (SMA) (g) and were immunonegative for CD34 (h) (positive control blood vessels – green arrow).
DISCUSSION
DFSP illustrates approximately 1.8–6% of STSs and 0.1% of all malignancies. DFSP occurs in nearly 0.8–5 cases/million persons/year. DFSP is most commonly reported in the second and fifth decades, although it can occur at any age.[8] In the previous reports, there was a male predominance. However, in the latest literature, equal gender distribution is found.[9] In this case series, the age range was 33–52 years with an equal gender distribution.
The origin of DFSP is likely coincidental. The exact reason for the development of DFSP is not clear; however, previous trauma to the affected region can be a prelude. In this case series, the occurrence was incidental. The chromosomal translocation t(17;22) (q22;q13) is found in about 90% of cases, which results in COL1A1-PDGFB fusion, leading to continuous activation of the PDGFB receptor. This leads to the rapid proliferation of DFSP tumor cells.[5]
Imaging in STS is to detect the extent of lesion for planning surgery. Underlying fascial/compartmental infiltration can be easily detected by CT/MRI, better by MRI as evident in imaging of the four cases. With simple tumor resection, the mean recurrence rate of DFSP is approximately 20% and with Mohs micrographic surgery, it is <1%.[7] Hence, it has a good prognosis. Post-operative follow-up in the first 3 years is very important. Follow-up after 6 months showed recurrence of tumor in the same site in two of our cases, while the other two did not have any recurrence or new lesion. MRI showed a very important role in the early assessment of recurrent lesions as evident in case 3.
The imaging features for DFSP need to be carefully differentiated from other diseases, especially definitive benign lesions, for example, lipoma, liposarcoma, dermatofibroma, neurogenic tumor, and hemangioma.
Lipoma and liposarcoma usually occur in the subcutaneous tissue of limbs, showing fat density on CT, and high signal on T1- and T2WI. It enhances a little post-contrast image. A low signal is seen in the fat suppression sequences. Neurogenic tumors such as schwannomas and neurofibromas grow mainly along nerves. Schwannoma often shows cystic degeneration with heterogeneous enhancement. Hemangioma, a painless soft-tissue mass, shows calcification on CT. It is isointense on T1WI images and hyperintense on T2WI, with dots or lines of low signal signifying calcification or venous stones. Centric enhancement is observed on enhanced images.[7]
CONCLUSION
Knowledge of the variable imaging appearances of DFSP may help in the diagnosis of difficult and atypical case scenarios. USG, a bedside modality, can easily show skin and subcutaneous tissue involvement. CT can be helpful to decide the line of incision. By avoiding inadequate excision, local recurrence or metastasis can be avoided. MRI has a role in accurate pre-operative assessment, early assessment of recurrent lesions, follow-up, and diagnosis of atypical and difficult cases.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
References
- Progressive and relapsing dermatofibrosarcomas or fibrosarcomas of the skin. Ann Dermatol Venereol. 1924;5:545-62.
- [Google Scholar]
- About the nodular fibrosarcoma skin (dermatofibrosarcoma protruberans) Dermatol Z. 1925;43:1-28.
- [CrossRef] [Google Scholar]
- Dermatofibrosarcoma protuberans: Radiologic appearance. AJR Am J Roentgenol. 1994;163:391-4.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: A clinicopathological study of nineteen cases and review of world literature. Scand J Plast Reconstr Surg. 1983;17:247-52.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: Pathology, genetics, and potential therapeutic strategies. Ann Diagnos Pathol. 2016;25:64-71.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: A review of the literature. Dermatol Surg. 2012;38:537-51.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: Computed tomography and magnetic resonance imaging findings. Medicine. 2015;94:e1001.
- [CrossRef] [PubMed] [Google Scholar]
- Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56:968-73.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: A comprehensive review and update on diagnosis and management. Semin Diagnost Pathol. 2013;30:13-28.
- [CrossRef] [PubMed] [Google Scholar]







