Translate this page into:
Magnetic resonance imaging features of large joint tuberculous arthritis

*Corresponding author: Shalini Agarwal, Department of Radiodiagnosis, Pandit Bhagwat Dayal Sharma, Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India. dragarwalshalini@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agarwal S, Mohan L, Lamba P, Kumar S. Magnetic resonance imaging features of large joint tuberculous arthritis. Indian J Musculoskelet Radiol 2021;3:82-7.
Abstract
Objectives:
Large joint monoarticular tuberculous involvement is rare. It may not be associated with classical clinical features. Hence, it is difficult to differentiate from other conditions similarly involving the large joints. Our study aimed to study the characteristics of large joint monoarthritis on magnetic resonance imaging.
Material and Methods:
We reviewed the radiology database for large joint tubercular arthritis cases over 2 years. In total, there were 21 patients. Male: female ratio was 11:10. The mean age was 34.14 ± 15.82 years with a range of 8–57 years. We diagnosed tuberculosis (TB) based on histopathological examination or response to antitubercular therapy.
Results:
Knee was most frequently involved (47%; n = 10) followed by wrist and elbow in 3 patients each (14.28%). Concomitant active pulmonary TB was absent in all of our patients. Grade I synovial thickening was seen in eight patients, Grade II in four, and Grade III in seven. It was uniform in all the cases. Grade 1 bone marrow edema was seen in 06 patients, Grade III in 13, and none in 02. There was soft-tissue edema in 12 patients and soft-tissue collection in 2. Bone erosions were seen in 16 patients with rim enhancement in nine patients. Central erosions were seen in eight, while central and peripheral erosions in eight. On T1-weighted images, the signal intensity was hyperintense 10 and isointense in 11 patients. While on T2-weighted images, it was hyperintense in 10, isointense in nine, and hypointense in two patients.
Conclusion:
Large joint monoarticular tuberculous arthritis can present variably. Large erosions with rim enhancement, the signal intensity of synovium on T1 weighted and T2 weighted, uniformity of synovial thickening, and enhancement pattern of abscesses can help make a diagnosis.
Keywords
Monoarticular
Large joint
Tuberculosis
Magnetic resonance imaging
INTRODUCTION
Musculoskeletal tuberculosis (TB) accounts for 1–3% of all tubercular cases. While spinal involvement is seen in approximately 50% of these cases.[1] The knee and the hip joint are the most commonly affected large joints.[2]
It is challenging to diagnose tubercular arthritis of large joints clinically because of non-specific signs and symptoms. Furthermore, these are not usually associated with pulmonary TB. It may mimic other chronic inflammatory arthritic disorders. As a result, there is often a delay in diagnosis, which may result in severe joint destruction and joint deformity.[3] Only less than half of the cases may have active intrathoracic disease.
It is imperative to treat infectious arthritis with appropriate treatment to avoid serious disability. The diagnosis of tubercular arthritis should be considered if there is an insidious onset of disease, substantial osteopenia, minimal sclerosis, and relative preservation of joint space indicates tubercular arthritis.[4] Pyogenic arthritis, on the other hand, is more aggressive.[5] However, TB may also present a more virulent pattern of destruction.[6] There is a rapid and excellent response to antitubercular therapy (ATT); hence, it is to the patient’s benefit to make an early and accurate diagnosis. Magnetic resonance imaging (MRI) is the modality of choice to detect early changes of tubercular arthritis. On MRI images, the features of tubercular arthritis are synovitis, effusion, central and peripheral erosions, active and chronic pannus, abscess, bone chips, and hypointense synovium. A definitive diagnosis can only be made by isolating the causative organism from the synovial fluid or synovial biopsy.[7]
MATERIAL AND METHODS
We obtained permission for waiver of consent from the institutional ethical committee before progressing with this study.
We reviewed the radiology database for large joint tubercular arthritis cases over a 2-year period, that is, from June 2016 to December 2020. A total of 21 patients were diagnosed with a tuberculous infection of large joints. Male: female ratio was 11:10. The mean age was 34.14 ± 15.82 years with a range of 8–57 years. There was no history of tuberculous infection such as pulmonary TB preceding the joint involvement in any of the patients. We performed MRI on a 3T MR Scanner with a dedicated extremity coil. Imaging was performed using spin-echo T1-weighted coronal and axial images (TR range/TE range, 450–650/14–18), and fast spin-echo T2-weighted sagittal and axial images (TR range/ effective TE range, 3000–4000/80–128; echo-train length, 7–8). Fat-suppressed images were performed to evaluate bone marrow edema. We also performed post-contrast images following administration of IV administration of gadopentetate dimeglumine (Magnevist, Schering) at a dose of 0.1 mmol/kg. We diagnosed tuberculous arthritis on the basis of aspiration and histopathological examination. Further, clinical response and resolution of radiological findings following ATT for diagnosis were used to indicate tuberculous infection if the histopathological examination was not done or was negative.
Analysis
MRI images were evaluated by a radiologist with 20 years of experience. We analyzed the features on MRI on the basis of the classification suggested by Choi et al.[8] The analysis was carried out with regard to the following parameters: Pattern and grade of synovial thickening, bone marrow edema on T2W FS, perisynovial soft-tissue edema, grade of joint effusion, the size of bone erosions, rim enhancement of bone erosions, location of bone erosions, the intensity of synovium on T1-W and T2-W images with respect to muscle, and the presence of lymph nodes. We classified the synovial thickening after enhancement into four grades: Grade 0, 0–3 mm; Grade 1, 3.1–6 mm; Grade 2, 6.1–9 mm; and Grade 3, >9 mm. The size of the largest bone erosion was documented in the longest diameter and classified into five grades: Grade 0, no erosion; Grade 1, < 6 mm; Grade 2, 6–10 mm; Grade 3, 11–15 mm; and Grade 4, >15 mm on T1-weighted images [Figure 1]. We determined enhancement around bone erosion as either present or absent. We did not count the number of erosions. We classified the extent of bone marrow edema into four grades based ([maximal distance from articular margin to outer margin of signal change of bone marrow on the sagittal or coronal image/maximal diameter of the articular surface on axial image] × 100[%]); Grade 0, no edema; Grade 1, 1–25%; Grade 2, 26–50%; and Grade 3, > 50% [Figure 2]. The patient was considered to have osteomyelitis when there was a well-defined intraosseous abscess/ subperiosteal collection/soft-tissue collection in association with bone marrow findings [Figure 3]. Soft-tissue perisynovial edema was measured as the maximal vertical distance from the outer margin of the joint capsule to the outer margin of periarticular soft-tissue edema on the coronal or sagittal image and classified as follows: Grade 0, no edema; Grade 1, 0.1–1 cm; and Grade 2, > 1 cm [Figure 4]. Edema of bone marrow and soft tissue was evaluated on fat-suppressed T2-weighted sequences. Joint effusion was classified into four grades: Grade 0 (absent): No joint effusion, Grade 1 (mild): Effusion in one pocket/recess of joint, Grade 2 (moderate): Effusion in 2 or more pockets/ recesses, and Grade 3 (severe): Gross joint effusion. Signal intensity was characterized on T1 weighted and T2 weighted as hypointense, hyperintense, or intermediate. This was based on the comparison with the signal intensity of adjacent muscles.

- Non-contrast and contrast-enhanced axial images of a 51-year-old female patient who presented with pain and swelling of the Rt shoulder of 1 month duration. It reveals a moderate Grade 2 joint effusion with Grade 1 enhancing synovial thickening and Grade 1 erosion (arrow) with diffuse uniform rim enhancement along the posterolateral aspect of the humeral head.
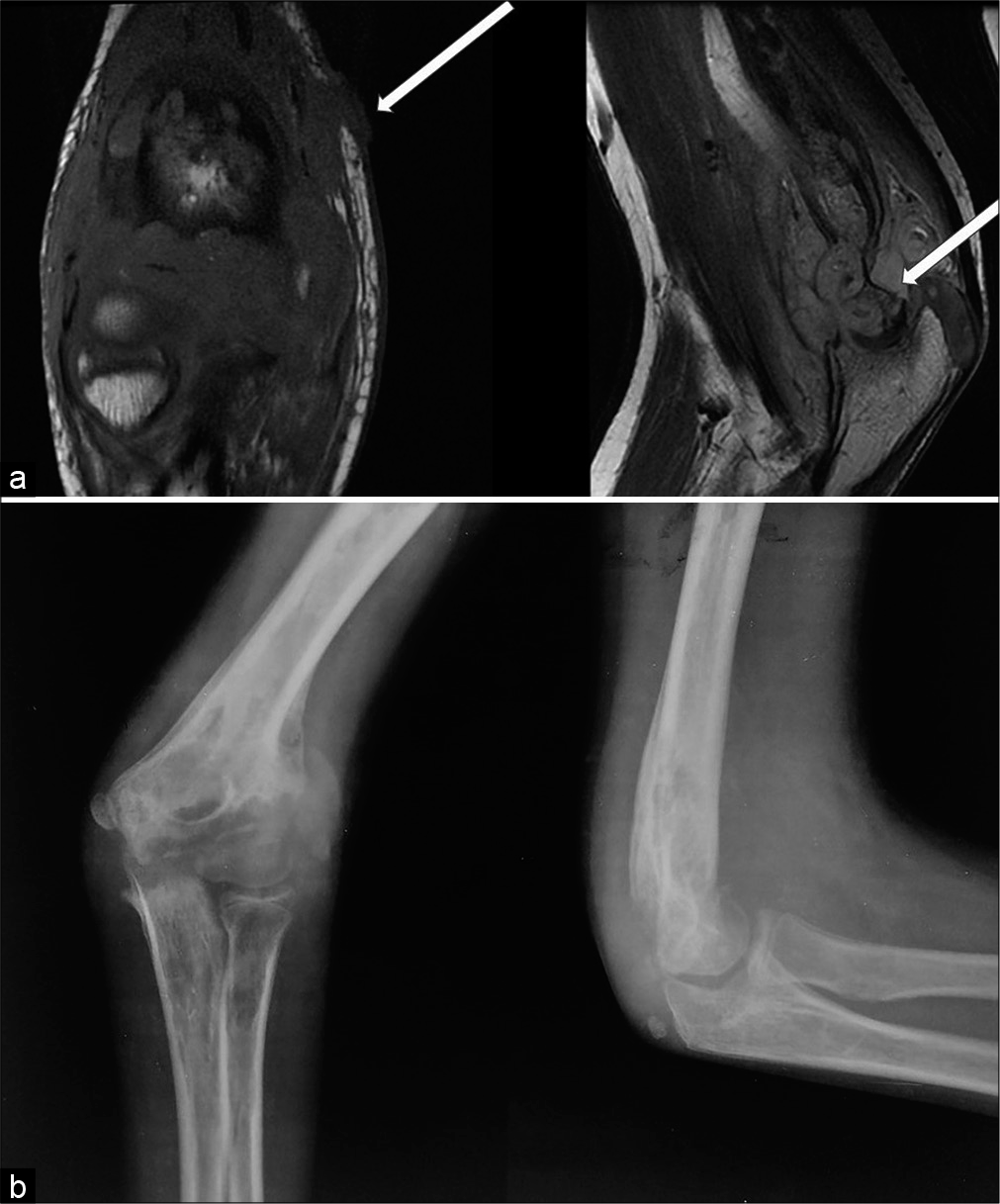
- (a) Non-contrast T1-weighted coronal and T2-weighted sagittal MR images of a 13-year-old male child who complained of swelling and pain over Rt elbow of 6 months duration reveals Grade 3 synovial thickening, Grade 3 erosion involving the trochlea (thick arrow), and an ulcer over the medial epicondyle (thin arrow). (b) Radiograph anteroposterior and lateral view of the Rt elbow revealed large erosions, periarticular osteopenia, soft-tissue edema.
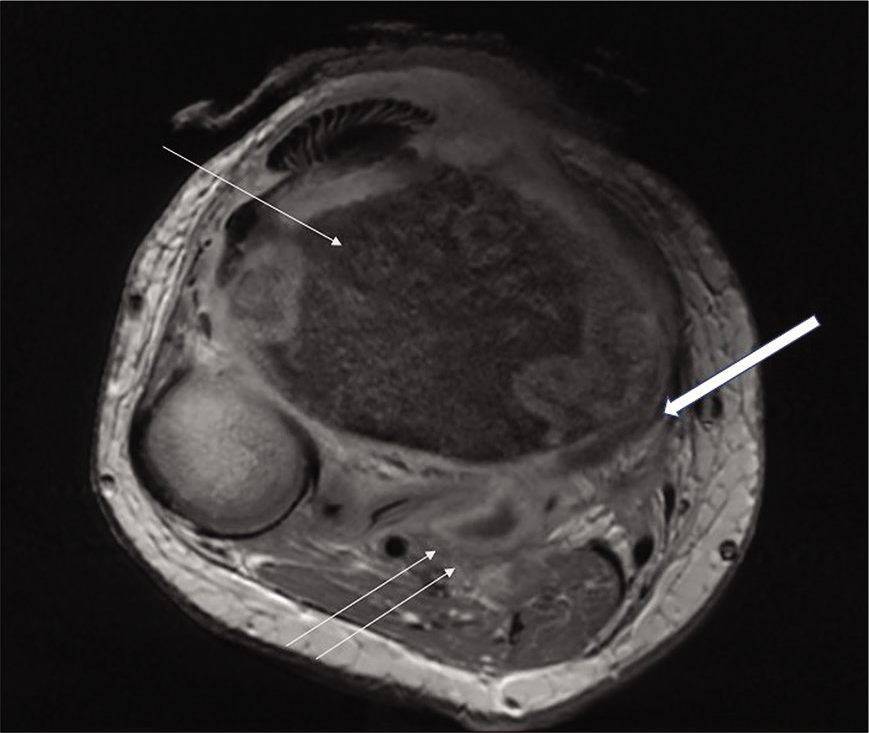
- Axial contrast-enhanced image of a 17-year-old female child shows altered signal intensity of tibia (thin arrow) with subperiosteal collection (thick arrow) and soft-tissue collections with uniformly enhancing thin walls (double arrows).
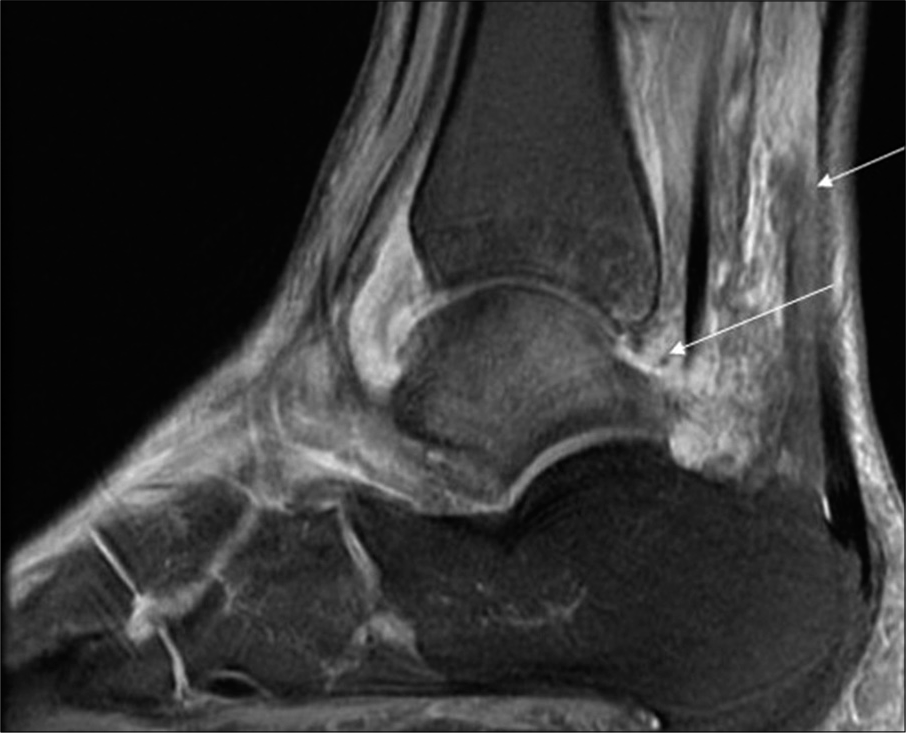
- Sagittal fat-suppressed T2-weighted magnetic resonance imaging image of the ankle of a 17-year-old female shows Grade 2 joint effusion and Grade 2 perisynovial edema (arrow).
Clinical/demographic data
Knee was most frequently involved in 47% of cases (n = 10), wrist in 14.28% (n = 3), elbow in 14.28% (n = 3), and 9% each for shoulder (n = 2) and hip joint (n = 1), ankle and midfoot (n = 2). We did not find bilateral joint involvement and concomitant pulmonary TB in any of our patients. The most common clinical presentation was pain in 13 patients, swelling in 17 patients, and limited range of motion in 20 patients. All patients received ATT. None of the patients required surgery.
MRI features
Various MRI features are shown in Table 1. Grade I synovial thickening was seen in 8 patients; Grade II in 4; and Grade 3 in 7. Grade I bone marrow edema was seen in 6; Grade III in 13, and no bone marrow edema in 2 [Figure 5]. There was no soft-tissue edema in 9 patients, Grade 1 in 8, and Grade II in 4 patients. There was no joint effusion in 3 patients, Grade 1 in 4, and Grade 2 in 14. Soft-tissue collection was seen in 1 patients of the wrist joint and 1 of the elbow joint. There were no erosions in 5, Grade 1 in 2, and Grade 2 in 13. Rim enhancement of these erosions was seen in 09 patients [Figure 6], and no rim enhancement in 12 patients. Central erosions were seen in 08, while central and peripheral erosions were also seen in 08. Signal intensity on T1-weighted images was hyperintense compared to muscles in 10 patients, while it was isointense in 11 patients. While on T2-weighted images, it was hyperintense in 10 patients, isointense in 09, and hypointense in 02 [Figure 6].
| S. No | Sex | Age | Joint involved | Synovial thickness (Grades) | Bone marrow edema/osteomyelitis (Grades) | Perisynovial/soft-tissue edema (Grades)/collections | Joint effusion (Grades) | Bone erosion (Grades) | Rim enhancement | Location of erosion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | M | 48 | Knee | 1 | 1 | 2 | 2 | 3 | Y | C |
| 2. | F | 20 | Knee | 2 | 3 | 0 | 2 | 0 | N | N |
| 3. | F | 8 | Wrist | 2 | 3 | 0 | 0 | 2 | Y | C&P |
| 4. | F | 51 | Shoulder | 1 | 3 | 1 | 2 | 2 | Y | C&P |
| 5. | F | 55 | Knee | 0 | 3 | 1 | 2 | 0 | N | N |
| 6. | F | 17 | Knee | 1 | 1 | 1 | 2 | 2 | N | C |
| 7. | M | 45 | Midfoot | 3 | 3 | 1 | 1 | 2 | Y | C&P |
| 8 | M | 44 | Hip | 1 | 1 | 0 | 1 | 2 | Y | C |
| 9 | M | 57 | Knee | 3 | 1 | 2 | 2 | 2 | Y | C |
| 10 | M | 13 | Elbow | 3 | 3 | 2 | 1 | 2 | Y | C&P |
| 11 | M | 32 | Elbow | 3 | 3 | 0 | 2 | 2 | N | C&P |
| 12 | M | 45 | Shoulder | 3 | N | 0 | 2 | 1 | N | C |
| 13 | F | 23 | Wrist | 0 | 3 | 1 | 0 | 0 | N | N |
| 14 | F | 11 | Knee | 1 | N | 0 | 2 | 0 | N | N |
| 15 | M | 37 | Knee | 1 | 3 | 1 | 2 | 2 | N | C&P |
| 16 | F | 34 | Knee | 3 | 3 | 1 | 2 | 2 | Y | C&P |
| 17 | M | 45 | Knee | 1 | 3 | 2 | 2 | 0 | N | N |
| 18 | F | 54 | Elbow | 3 | 1 | 0 | 1 | 1 | N | C |
| 19 | M | 38 | Wrist | 1 | 1 | 1 | 0 | 2 | Y | C |
| 20 | M | 23 | Knee | 2 | 3 | 0 | 2 | 2 | N | C&P |
| 21 | F | 17 | Ankle | 2 | 3 | 0 | 2 | 2 | N | C |
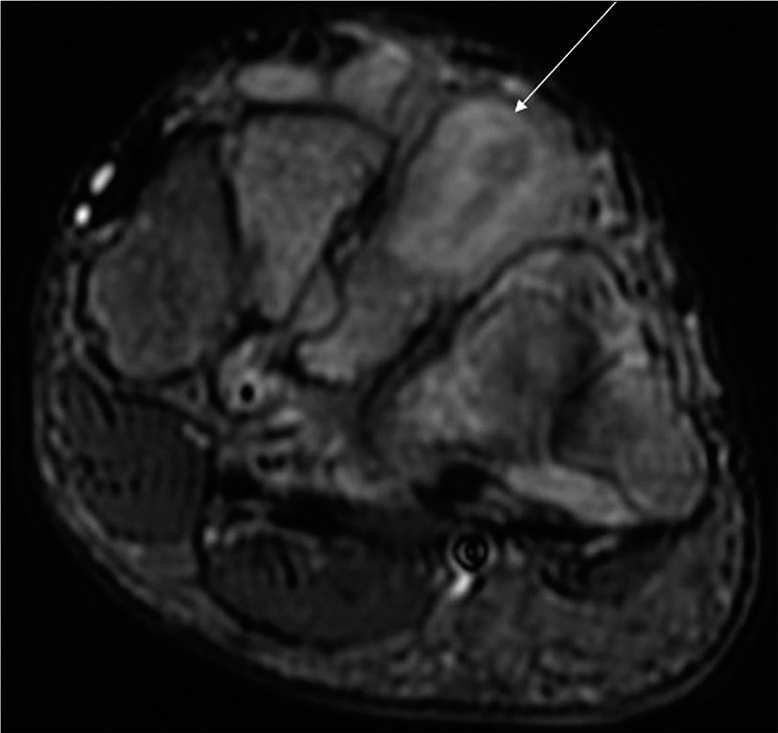
- Axial T2-weighted fat-suppressed image of a 45-year-old male patient with a histopathologically proven case of the midfoot tubercular involvement shows evidence of osteomyelitis involving the third metatarsal (arrow).
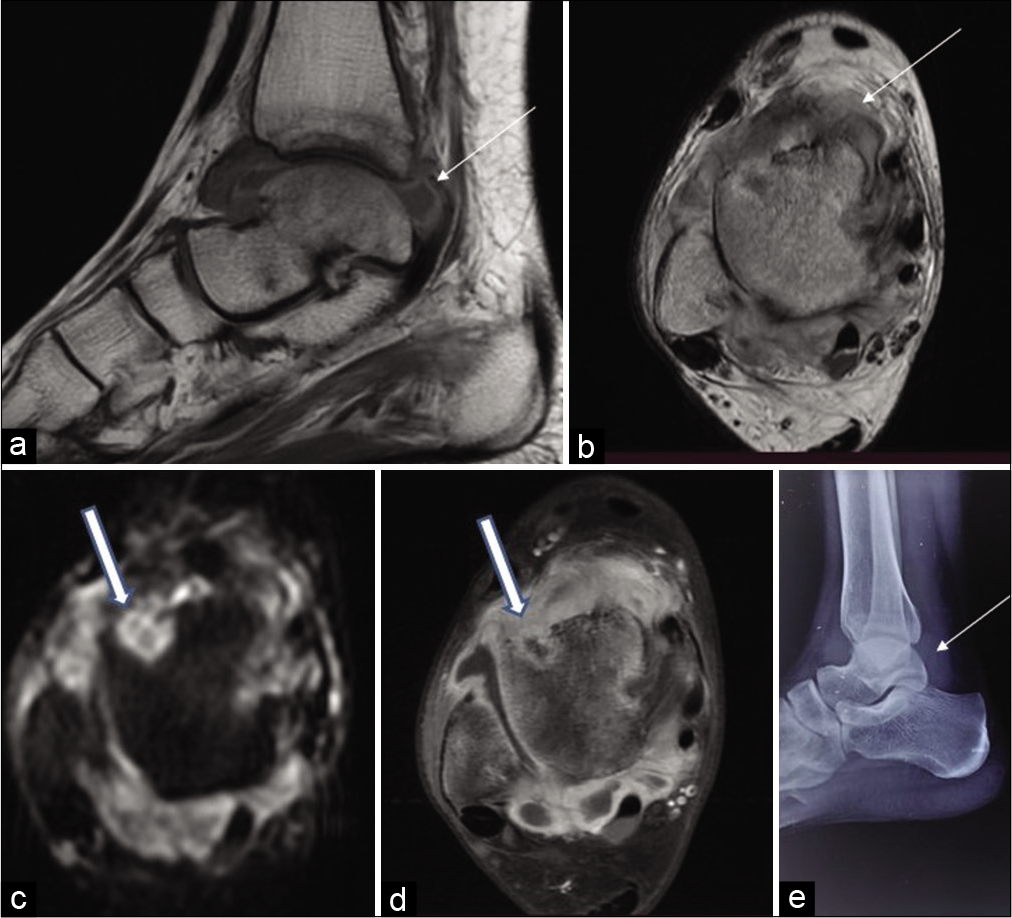
- Sagittal T1-weighted (a) and axial T2-weighted (b) MR images of the ankle joint of a 17-year-old female reveal uniform Grade 1 synovial thickening that is hyperintense on T1-weighted images (thin arrow) and hypointense on T2-weighted images (thick arrow). Axial diffusion-weighted image (c) and axial contrast-enhanced images (d) reveal erosions with enhancing rim and restriction on diffusion-weighted image (thick arrow). Radiograph lateral view (e) reveals thickened synovium (thin arrow).
DISCUSSION
TB retains its importance in the modern medical world because of its high prevalence in immunocompromised patients and the development of multidrug-resistant strains.[9] Early diagnosis is challenging as tubercular arthritis can present variably, and it may simulate other chronic inflammatory disorders. Many times it is diagnosed as post-traumatic arthritis.[10] As a result, there is a delay in diagnosis and severe joint destruction with joint deformity.[3]
It is a treatable condition, and if diagnosed early, can show good results.[9] In view of good resolution, MRI is superior to other modalities in the diagnosis of this condition. It can demonstrate joint fluid and synovial hypertrophy, pannus, bone erosions, cartilage destruction, associated osteomyelitis, etc.
Tubercular arthritis is predominantly a synovial disease and usually presents as monoarthritis. Synovitis is the most common finding. Prakash et al. performed an MRI on 12 patients with tuberculous arthritis of the elbow joint and found synovial involvement in all (12/12) patients.[11] There were similar findings in our study.
In the available literature, the hypointensity of synovium on T2-weighted images has been found to be typical of tubercular arthritis. Sawlani et al.[9] and Sanghvi et al.[12] found hypointense synovium on T2-weighted imaging in 40% and 75% of cases in their study. Sanghvi et al. found low signal intensity in 9/15 patients and intermediate signal on T2-weighted images in six patients; however, synovial proliferation was found in all (15/15) patients. Hypointense synovium was reported on T2-weighted images by Hsu et al.[13] and Prakash et al.[10] in 3 (37.5%) and 5 (46%) patients, respectively. A hypointense synovial signal was seen in only 2 (9.5%) of our patients, while in the rest, it revealed iso- to hyper-intensity. Pannus on MRI appears as intermediate to low signal intensity on TI- weighted and intermediate to high signal intensity on T2-weighted images.[8] Suh et al.[14] reported that caseous necrosis has an intermediate signal on T2 weighted.
Choi et al. evaluated 63 joints with clinically or pathologically proven RA involving 36 joints and tuberculous arthritis involving 27 joints.[8] Non-uniform synovial thickening was noted in 72–86% of rheumatoid cases and 45–55% cases of tubercular arthritis. Grade 0 synovial thickening was observed in 44–55% of tubercular cases. Grade 3 was observed in 22–44% cases of RA and 7–14% cases of tubercular arthritis.[8] Two (9.5%) of our patients revealed Grade 0 synovial thickening, while 5 (23.8%) had Grade 3 synovial thickening. All patients in our study had uniform synovial thickening.
Erosion is the second most common finding in tuberculous arthritis and is seen in approximately 70% of cases. They are central as well as peripheral in location. They appear hypointense on T1-weighted and T2-weighted images.[9] Hsu et al.[13] reported bone erosions in 7/8 patients. Prakash et al.[11] reported erosions in all (12/12) patients. In contrast, Sanghvi et al.[12] reported them in 5/15 patients. According to a study by Choi et al.,[8] larger erosions with rim enhancement favor tubercular arthritis, while erosions with thicker irregular enhancement favor pyogenic arthritis. There were bone erosions in 16 (76.19%) of our patients. Out of these, 14 had Grades 2 and 3 erosions.
Bone chips are considered a distinguishing feature of tubercular arthritis. However, these are rare and have been previously reported in spinal TB and not in peripheral joints.[9] Bone chips were not seen in any of our patients.
Abscesses are characteristics of infective arthritis, and they appear hyperintense with a hypointense rim on T2-weighted and hypointense with a hyperintense rim on T1-weighted images. On postcontrast images, they show uniform rim enhancement. These are differentiated from abscesses associated with septic arthritis, which reveal marked periarticular inflammation.[9] Sanghvi et al.[12]and Prakash et al.[11] found large, thin-walled abscesses in the surrounding soft tissue in 3/15 patients and 9/12 patients, respectively. Hong et al.[15] found abscesses with a thin and smooth wall in 16/16 patients with tubercular arthritis. There were soft-tissue abscesses in 2 of our patients. All had thin and smooth walls.
Karchevsky et al.[16] found perisynovial edema in 84% of their septic arthritis patients. In their study, perisynovial edema, synovial enhancement, and joint effusion had the highest correlation with the diagnosis of septic arthritis. We found Grades 1–2 perisynovial edema in 57.14% of our patients with tubercular arthritis.
In cases of involvement of hand and wrist, tubercular tenosynovitis is a common finding.[9] Hsu et al.[12] evaluated eight patients with tubercular arthritis involving the wrist joint and found synovial thickening around the flexor and extensor tendons in all patients. Prakash et al.[11] did not find tenosynovitis in any of their 12 patients of elbow TB; however, they found tenosynovitis in 50% of their study population with ankle and foot TB.[17] There were two patients with ankle involvement and three with wrist involvement in our study. Only one patient with ankle involvement did not have tenosynovitis [Figure 7].
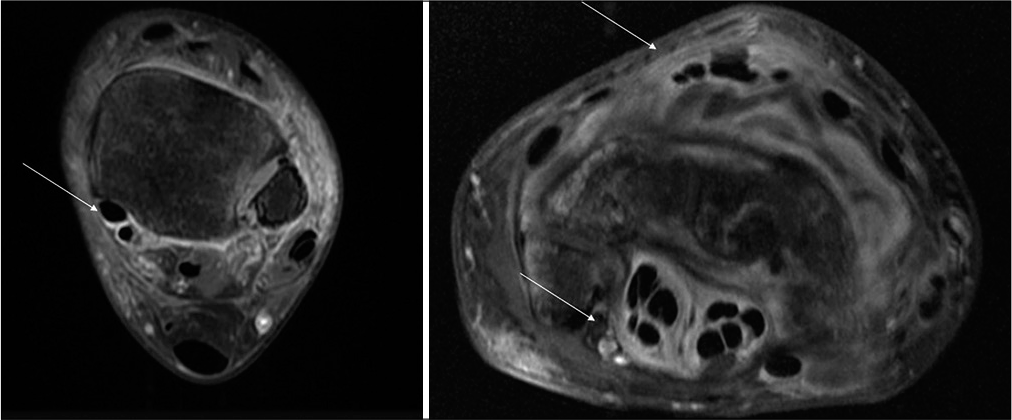
- Axial contrast-enhanced images of the ankle and wrist of a 17-year-old female patient and a 38-year-old male patient shows tenosynovitis of the flexor tendons of the foot and of the flexor and extensor tendons of the wrist.
Osteomyelitis of bone may be an associated finding. Sanghvi et al.[12] and Hsu et al.[13] found it in 3/15 and 6/15 of their patients, respectively. In our study, there were 3 (14.28%) patients with features suggestive of osteomyelitis. Sanghvi et al.[12] found subchondral bone marrow edema in 9/15 patients without features of osteomyelitis. There were 10 (47.62%) such patients in our study. Choi et al.[8] found Grade 3 bone marrow edema in 44–60% of tubercular arthritis patients and 25–41% of RA patients. There was Grade 3 bone marrow edema in 13/21 of our patients. Hong et al.[15] found marrow signal intensity abnormality in 17/29 patients of tubercular arthritis.
CONCLUSION
Large joint monoarticular tubercular monoarthritis is rare but not uncommon in populations with a high prevalence of this disease. It is very easily confused with other causes such as pyogenic arthritis and rheumatoid arthritis. Early diagnosis and proper treatment are important to prevent joint destruction and permanent disability. Large erosions with rim enhancement, the signal intensity of synovium on T1 weighted and T2 weighted, uniformity of synovial thickening, and enhancement pattern of abscesses on contrast-enhanced MRI images can help in making a diagnosis.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Tuberculosis of the spine. Controversies and a new challenge. Spine (Phila Pa 1976). 1997;22:1791-7.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of extrapulmonary tuberculosis. Radiographics. 2000;20:471-88.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous arthritis of the knee: MR findings. AJR Am J Roentgenol. 1993;160:664.
- [CrossRef] [Google Scholar]
- Rheumatoid arthritis and tuberculous arthritis: Differentiating MRI features. AJR Am J Roentgenol. 2009;193:1347-53.
- [CrossRef] [PubMed] [Google Scholar]
- MRI features of tuberculosis of peripheral joints. Clin Radiol. 2003;58:755-62.
- [CrossRef] [Google Scholar]
- Two cases of chronic arthritis of the forearm due to Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 1998;17:344-8.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging findings in tubercular arthritis of elbow. Clin Imaging. 2016;40:114-8.
- [CrossRef] [PubMed] [Google Scholar]
- MRI features of tuberculosis of the knee. Skeletal Radiol. 2009;38:267-73.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous infection of the wrist: MRI features. AJR Am J Roentgenol. 2004;183:623-8.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of tuberculous arthritis: Clinical and experimental studies. J Magn Reson Imaging. 1996;6:185-9.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous versus pyogenic arthritis: MR imaging evaluation. Radiology. 2001;218:848-53.
- [CrossRef] [PubMed] [Google Scholar]
- MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am J Roentgenol. 2004;182:119-22.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging evaluation of tubercular arthritis of the ankle and foot. Acta Radiol. 2015;56:1236-41.
- [CrossRef] [PubMed] [Google Scholar]






