Translate this page into:
Multimodality imaging in shoulder arthroplasties Part 1: Biomechanics, indications, preoperative imaging, and types of arthroplasties – A pictorial review

*Corresponding author: Harun Gupta, Department of Musculoskeletal Radiology, Leeds Teaching Hospitals, Leeds, West Yorkshire, United Kingdom. harungupta@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sarvesvaran M, Thaker S, Srinivasan S, Brar RS, Bhatt R, Gupta H. Multimodality imaging in shoulder arthroplasties Part 1: Biomechanics, indications, preoperative imaging, and types of arthroplasties – A pictorial review. Indian J Musculoskelet Radiol 2021;3(1):24-9.
Abstract
Shoulder biomechanics, shoulder arthroplasty indications and types and appropriate pre-operative imaging are discussed in part one of this two-part pictorial review. Shoulder biomechanics in severe osteoarthritis and following reverse shoulder arthroplasty are represented graphically, with discussion on the principles of reverse shoulder arthroplasty. Case examples are utilized to demonstrate the main indications for shoulder arthroplasty.
The decision of whether to proceed with arthroplasty and the arthroplasty type is heavily influenced by the pre-operative imaging. Factors for type of arthroplasty include arthroplasty indication, the integrity of the deltoid and rotator cuff musculature, and the amount of glenoid and humeral head bone stock. The key findings to look for and comment on across a range of imaging modalities are reviewed, using multiple cases including plain radiography, CT, ultrasound, and MRI. Examples of arthroplasty options are provided including humeral head resurfacing arthroplasty, hemiarthroplasty, anatomical total shoulder arthroplasty, and reverse total shoulder arthroplasty.
A good understanding of the above principles is crucial for the radiologist to interpret pre-operative imaging correctly and aid in surgical planning.
Keywords
Shoulder arthroplasty
Shoulder replacement
Osteoarthritis imaging
Glenohumeral osteoarthritis
Joint degeneration
INTRODUCTION
Shoulder arthroplasty remains the mainstay of surgical treatment for numerous indications after failure of conservative management of the painful shoulder with severely restricted movements. Musculoskeletal radiologists play a key role by strategic use of various imaging methods. This helps the orthopedic surgeon, by providing crucial pre- and peri-operative information and monitors post-operative patient progress including complications.
We have divided shoulder arthroplasty imaging into two parts for easier understanding. In the first part, we describe the biomechanics of the degenerated and implanted shoulder, discuss indications, and focus on appropriate pre-operative imaging. In part two, we revisit shoulder arthroplasty complications before introducing new perioperative concepts, including 3D-printed customized glenohumeral implants, pre- and intra-operative CT-guided navigation and emerging arthroplasty techniques. They minimize human error and arguably improve accuracy of implant placement, which in turn should benefit our patients.
BIOMECHANICS
While hemiarthroplasty and anatomical total shoulder arthroplasty (TSA) replicate anatomy and biomechanics of the normal shoulder joint, reverse TSA (RTSA) aims to reverse the glenohumeral anatomy to exploit the use of deltoid to provide the function of the deficient rotator cuff. Grammont, in 1985, has described four cardinal principles of RTSA: (1) Medialized center of rotation, (2) distalizing and therefore tensioning the deltoid onto the humerus, (3) stable center of rotation, and (4) a semi-constrained prosthesis with a larger arc of motion [Figure 1]. These principles have revolutionized prosthesis designs and widened indications for RTSA.[1]
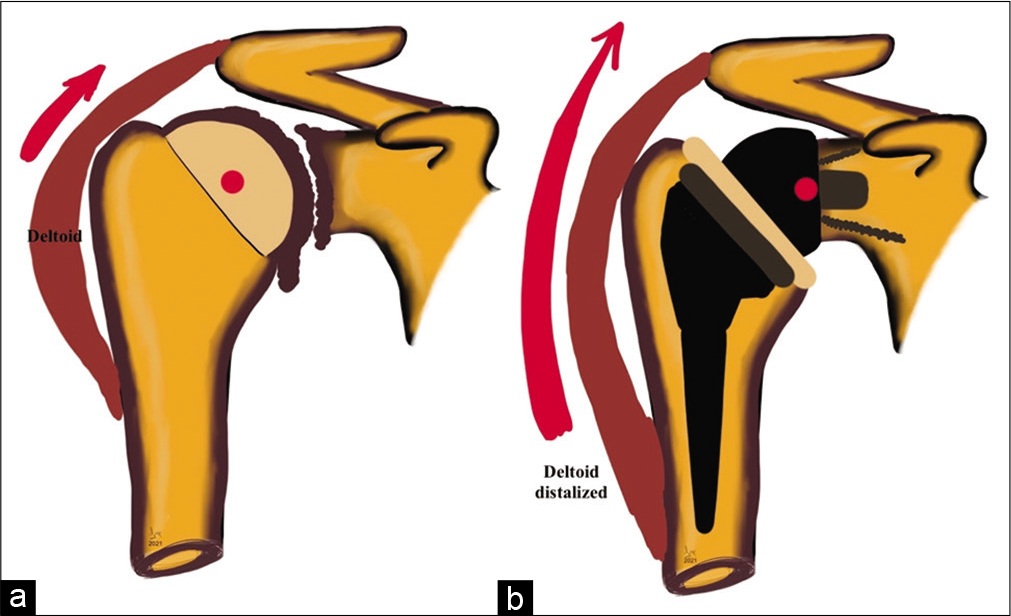
- Graphical representation of shoulder biomechanics in (a) severe glenohumeral osteoarthritis demonstrating near-complete loss of joint space, marginal osteophytes at inferior glenoid and humeral head-neck junction, normal deltoid pull, and deficient rotator cuff as seen by superior migration of the humeral head and (b) following reverse total shoulder arthroplasty showing post-operative medialization of the center of rotation of the glenohumeral joint (red dot), distalization of the deltoid attachment increasing lever arm length (red arrow), and recruiting more deltoid fibers to increase shoulder abduction and semi-constrained nature of the prosthesis.
INDICATIONS
Shoulder arthroplasty indications are similar to those in other joints, including osteoarthritis, inflammatory arthropathy, and avascular necrosis of the humeral head. Indications unique to the shoulder joint are rotator cuff arthropathy, complex proximal humerus fractures, shoulder arthroplasty revisions, and bone tumors involving the proximal humerus.[2]
For accurate assessment of glenohumeral osteoarthritis, a dedicated Grashey view is essential for accurate assessment of the joint space [Figure 2]. The classic osteoarthritis findings are non-uniform joint space loss, osteophytosis, subchondral cystic change, and subchondral sclerosis [Figure 3a]. Uniform joint space narrowing, central erosive changes and absence of marginal osteophytes, and subchondral changes are usually features of an inflammatory arthropathy [Figure 3b]. One should remember that end-stage inflammatory arthropathy can demonstrate overlapping imaging features with primary osteoarthritis. Proximal humeral head fractures, not suitable for open reduction and internal fixation, are considered for treatment with arthroplasty [Figure 4]. The Neer classification is used to characterize the fracture pattern. Neer type 3 and 4 fractures typically result in arthroplasty in patients requiring activity preservation, who have preserved deltoid and subscapularis function.[3] Irreparable massive rotator cuff tears and associated severe rotator cuff arthropathy are also candidates for arthroplasty[2] [Figure 5]. Revision arthroplasties are increasingly performed as the number of total shoulder arthroplasties is increasing.[4] Indications for revision include primary procedures complicated by infection and superior prosthesis migration due to irreparable rotator cuff tears. Custom-designed prostheses can be utilized for malignant tumors of the humerus in highly specialized centers.[5]
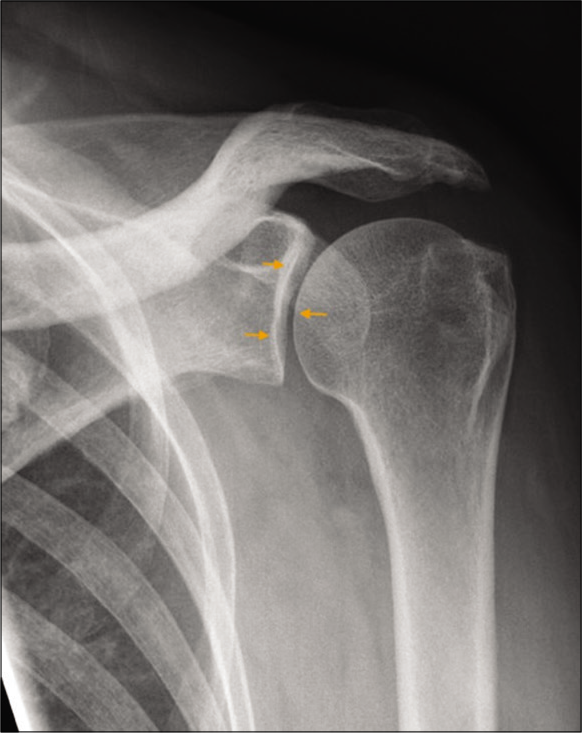
- A 43-year-old diabetic male presented with left shoulder pain and restriction of movements. True anteroposterior radiograph (Grashey view) of the left shoulder joint demonstrating a true orientation of the glenohumeral joint space (orange small arrows). Also note preserved acromiohumeral distance and absence of osteophytes or subchondral changes.
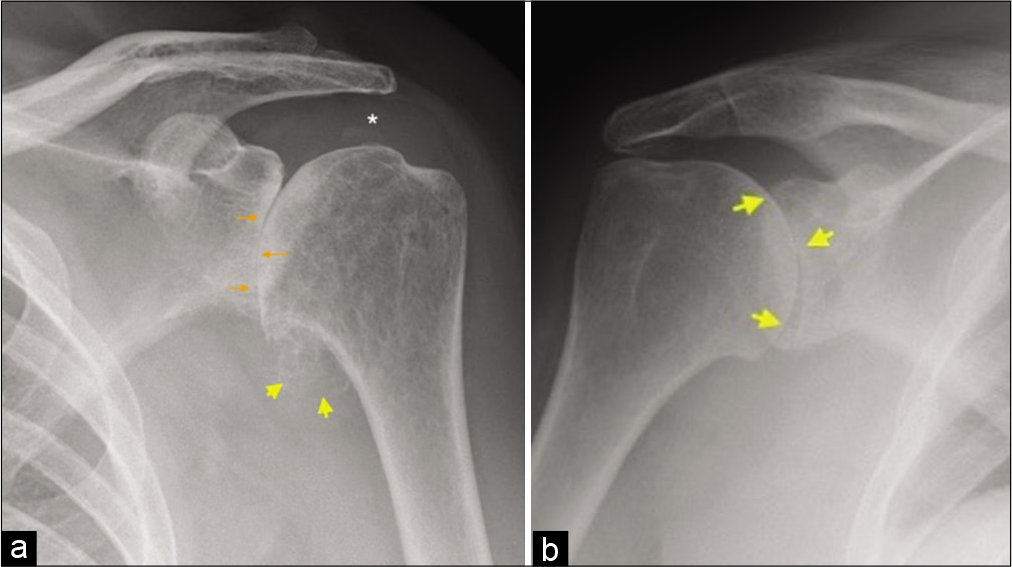
- (a) A 67-year-old female presented with the left shoulder pain and movements restriction. True anteroposterior radiograph (Grashey view) demonstrating a complete loss of glenohumeral joint space, subchondral sclerosis and early subchondral cystic changes (orange arrows), sizeable humeral osteophytes in the region of axillary recess (yellow arrow heads), and preserved acromiohumeral distance (asterisk) consistent with findings of severe glenohumeral osteoarthritis of primary degenerative nature and (b) a 44-year-old female presented with multiple joint pain and predominant symptoms in the right shoulder joint. AP radiograph of the right shoulder joint demonstrating symmetrical joint space loss (yellow short arrows) with conspicuous absence of osteophytes and other subchondral changes indicating inflammatory arthropathy. The patient had rheumatoid factor and anti-CCP antibody-positive rheumatoid arthritis.
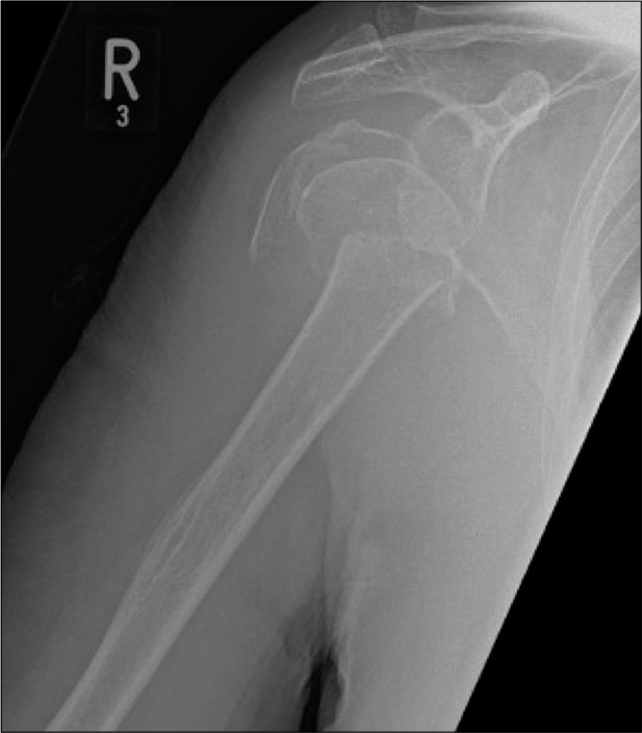
- An 81-year-old male with a history of fall down stairs. AP radiograph of the right shoulder joint demonstrates complex multifragmentary proximal humeral fracture. Also appreciate severe osteopenic nature of visualized bones.
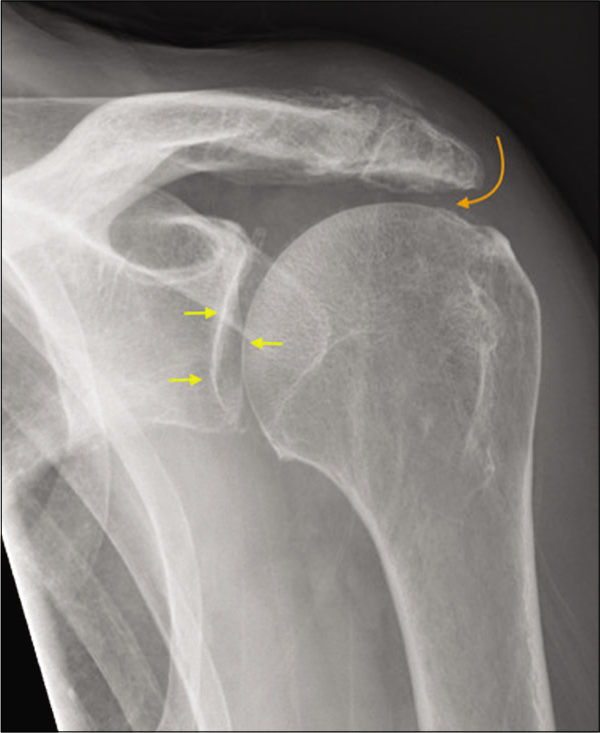
- A 55-year-old manual worker presented with shoulder pain predominantly following overhead activities. Grashey view of the left shoulder joint depicting normal glenohumeral joint space (yellow short arrows) and marked narrowing of the acromiohumeral distance (curved orange arrow), degenerate acromioclavicular joint, and absence of osteophytes consistent with early rotator cuff arthropathy. The patient had a history of secondary subacromial impingement and partial-thickness cuff tear which has progressed to medially retracted full-thickness rotator cuff tear.
PRE-OPERATIVE IMAGING AND ITS UTILITY IN ARTHROPLASTY DECISION-MAKING
Radiography remains the first-line imaging method to confirm and categorize indications of shoulder arthroplasties. It can assess glenoid morphology and bony remodeling besides confirming the presence of degenerative disease [Figure 6]. Radiography has been superseded by the increasing use of CT.[6] Furthermore, superior migration of the humeral head on radiographs or CT with a reduced acromiohumeral distance of <7 mm implies rotator cuff failure.[7] In advanced cases of rotator cuff arthropathy, one can readily assess femoralization of the humeral head and acetabularization of the acromion undersurface[2] [Figure 7].
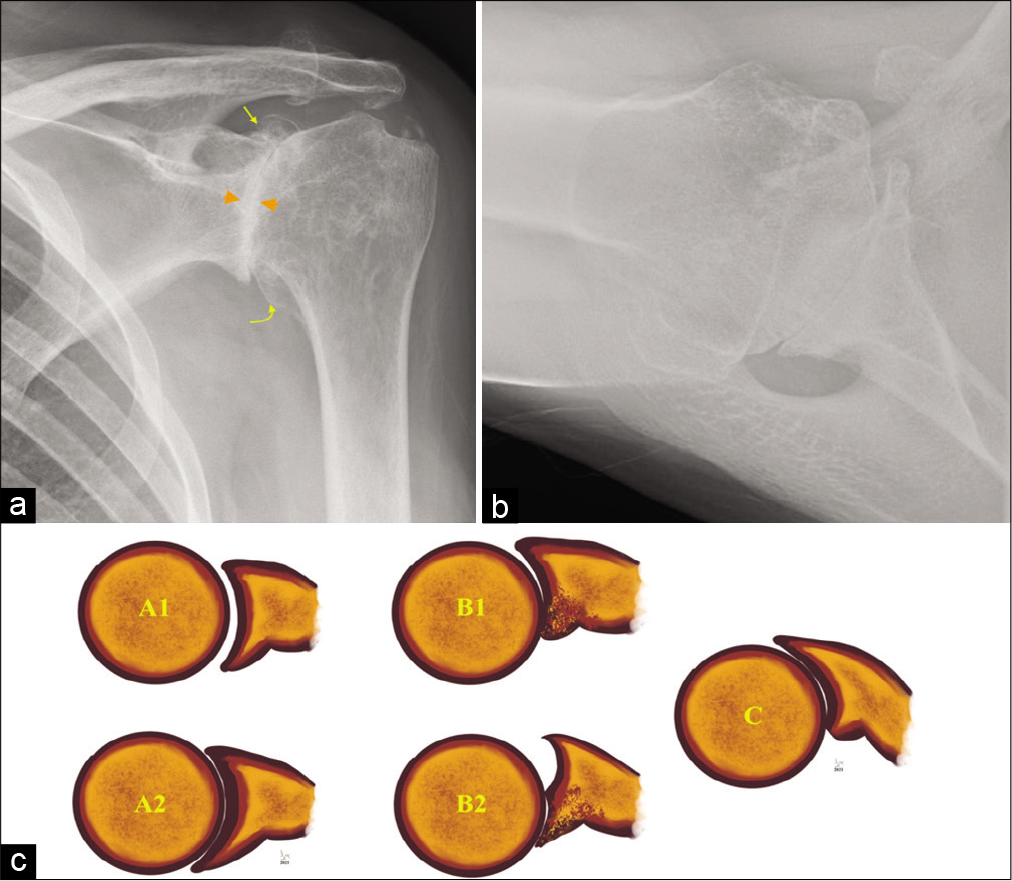
- An 80-year-old male presented with long-standing history of the left shoulder pain and movement restriction. (a) Grashey view of the left shoulder joint showing bone-on-bone appearance with complete loss of joint space and subchondral changes (orange arrowheads) and circumferential marginal osteophytes involving glenoid (straight yellow arrow) and humeral head (curved yellow arrow) and (b) axillary view demonstrating glenoid remodeling and change in the glenoid morphology besides confirming severe osteoarthritis. (c) Graphical representation of the Walch classification extensively used by shoulder surgeons preoperatively to select surgical procedure and glenoid prosthesis. A1 (early) and A2 (established) concentric glenoid cartilage loss, most commonly seen following inflammatory arthropathy whereas B1 (early) and B2 (advanced) eccentric, posterior predominant glenoid cartilage loss with remodeling of the articulating glenoid is predominantly seen in primary degenerative osteoarthritis. Glenoid retroversion of more than 25° with posterior humeral head subluxation (c) requires additional bone graft or modified baseplate to augment posterior glenoid. This classification has further extended describing anterior glenoid remodeling and advanced posterior cartilage loss.
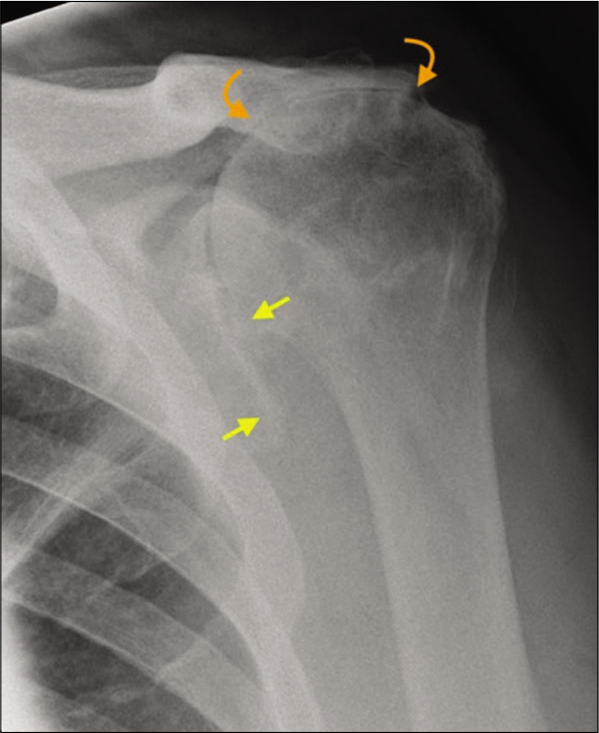
- A 72-year-old male presented with long-standing history of rotator cuff tear and severe loss of the left shoulder function. There is complete loss of acromiohumeral distance due to superior migration of the humeral head (yellow arrows) resulting in bony remodeling of the undersurface of the acromion process termed as “acetabularization” of the acromion (curved orange arrows). These appearances are characteristic of longstanding failed rotator cuff and secondary advanced rotator cuff arthropathy.
CT remains a cornerstone imaging technique used in all pre-operative cases for evaluating subchondral changes, their location, the adequacy of glenoid bone stock, and glenoid version [Figure 8]. Modern shoulder arthroplasty planning software require CT scan as per their protocol. It assists the surgeon in determining operative suitability and use of appropriate implant and designing a targeting jig. CT is an essential part of 3D printing of tailored implants, with CT navigation now widely used by shoulder surgeons globally, further discussed in part 2.
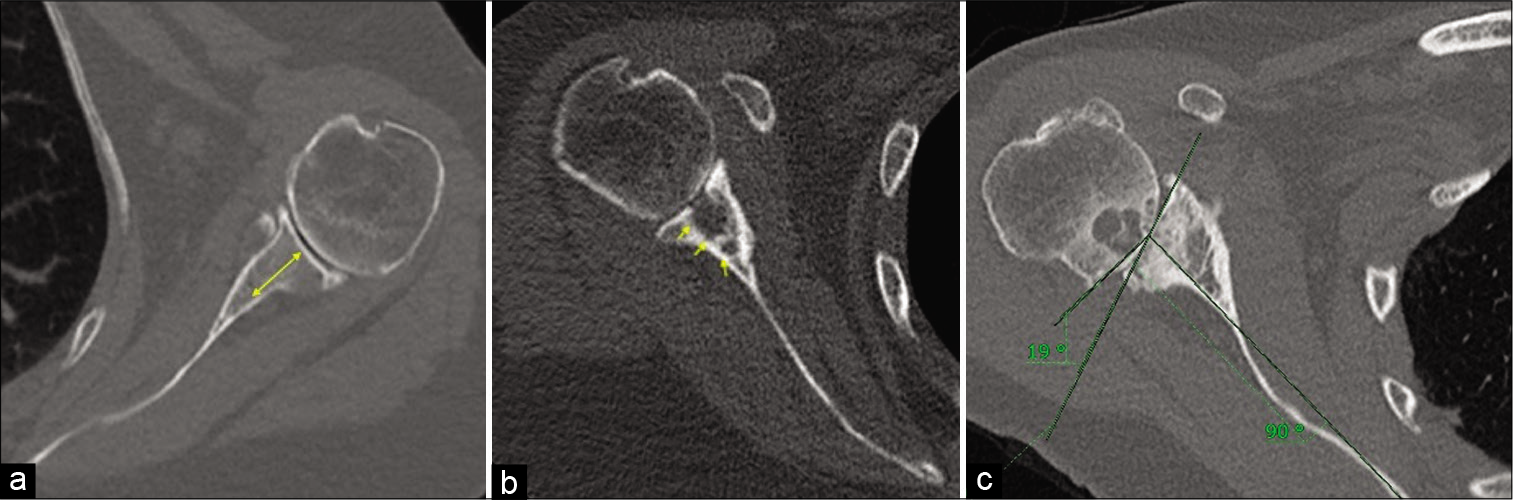
- (a) A 66-year-old female with left shoulder osteoarthritis. True axial image of the CT scan (bone kernel) depicting adequate glenoid bone stock. The glenoid bone stock is measured at the center of the glenoid in a reformatted true axial image from articulating surface of the glenoid to the scapular neck and should be devoid of large intraosseous cystic changes. Good glenoid bone stock is necessary for adequate glenoid component and screw purchase to reduce chances of prosthesis loosening. (b) A 73-year-old female with severe right shoulder osteoarthritis. Axial CT scan image in a bony kernel showing large intraosseous cystic changes resulting in poor glenoid bone stock. (c) A 69-year-old female with right shoulder osteoarthritis. Reformatted true axial image of the right shoulder joint in a bony kernel showing a Friedman’s technique of the glenoid version measurement. Friedman’s line is measured from the medial tip of the scapular up to the midpoint of the articulating glenoid and another line is drawn perpendicular to it. It is also known as scapular axis. Another line connecting the anterior and posterior margin of the glenoid is drawn. The angle between the scapular axis and the plane of the glenoid is taken as angle of retroversion (in our case 19°). Also appreciate for humeral head bone stock which is surgically less relevant for planning reverse total shoulder arthroplasty except in cases where humeral head graft is needed to augment the glenoid bone stock (e.g., BIO RSA – Bony Increased Offset Reversed Shoulder Arthroplasty).
Sonography allows for dynamic assessment of the rotator cuff integrity [Figure 9], tendinopathy, and tears [Figure 10] with increasing sensitivity and specificity approaching MRI.[8] It can also play a key role in the preoperative imaging of rotator cuff arthropathy.
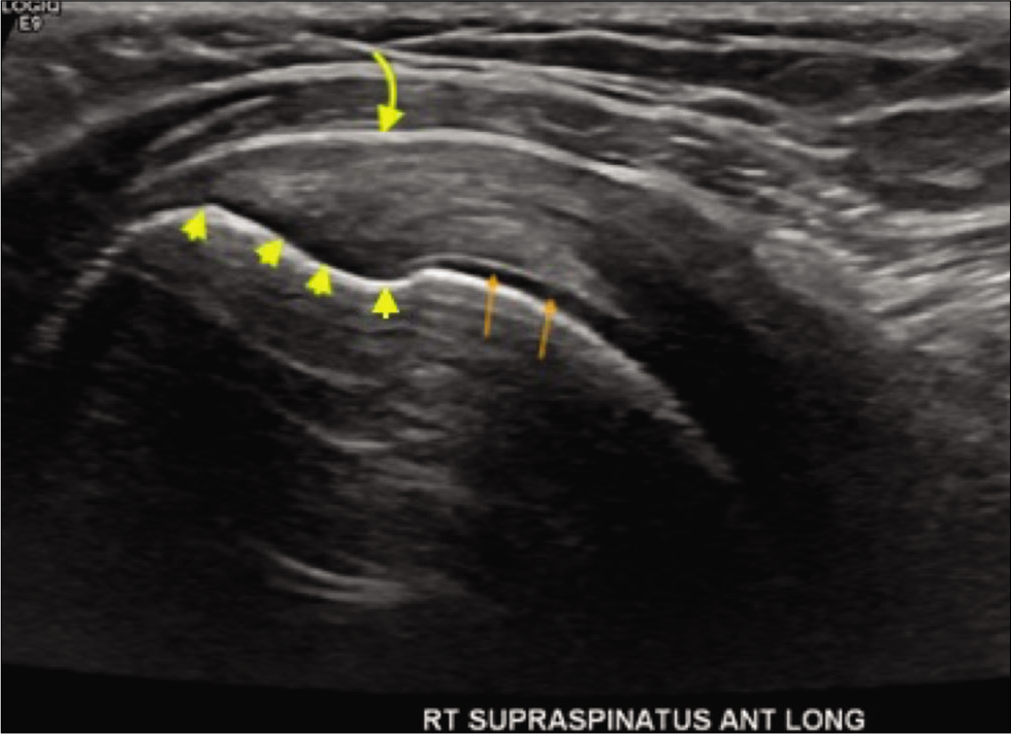
- A 34-year-old female presented with the right shoulder pain while raising arm overhead. Grayscale ultrasound image through the long axis of the supraspinatus enthesis depicting supraspinatus footprint (yellow arrowheads) with underlying normal subchondral bone, adjacent pristine humeral head cartilage (orange arrows), and normal appearances of subacromialsubdeltoid bursa (curved yellow arrow).
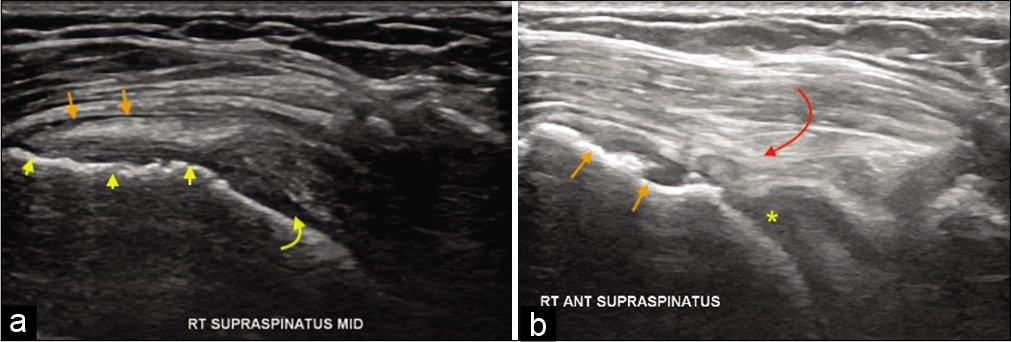
- A 55-year-old female with the right shoulder pain and impingement symptoms. (a) Grayscale ultrasound demonstrating partial-thickness tear of the distal supraspinatus with cortical irregularity at its enthesis (yellow arrowheads), irregularity of the humeral head cartilage (yellow curved arrow), and subacromial subdeltoid bursitis (orange short arrows). Changes in humeral head cartilage may indicate heralding rotator cuff arthropathy. Follow-up ultrasound after 6 years. (b) In the same, patient showing full-thickness tear with remarkably thin distal tendon (curved red arrow), osteophytes at the supraspinatus enthesis (orange short arrows, and completely degenerated humeral head cartilage replaced by joint effusion (yellow asterisk). The patient has had severe fatty atrophy of the supraspinatus (shown below in MRI) and advanced rotator cuff arthropathy on the radiograph.
Besides establishing the diagnosis of shoulder osteoarthritis and rotator cuff tears, MRI is the best modality for assessing for atrophy and fatty infiltration of the rotator cuff [Figure 11]. Hence, the MRI has a prognostic value when used preoperatively and guide shoulder surgeons to choose the appropriate technique. Goutallier et al. classification is the most commonly used semi-quantitative classification system for fatty atrophy[9] which relies on the muscle-to-fat ratio of primarily the supraspinatus and infraspinatus musculature [Figure 12].
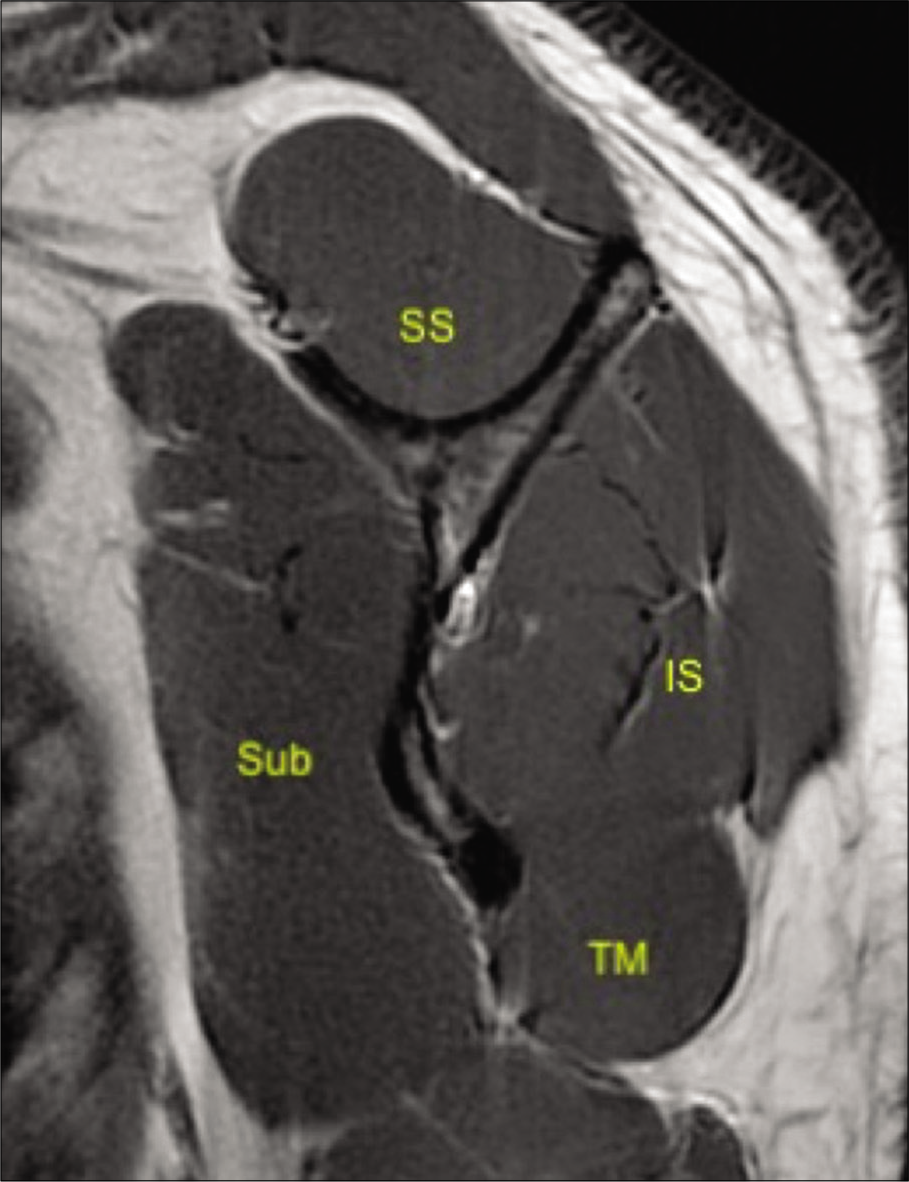
- A 33-year-old male volunteer without any shoulder symptoms. T1-weighted sagittal image at the level of the scapular neck demonstrating normal appearances of rotator cuff comprising of subscapularis (Sub), supraspinatus (SS), infraspinatus (IS), and teres minor (TM) and their myotendinous junctions.
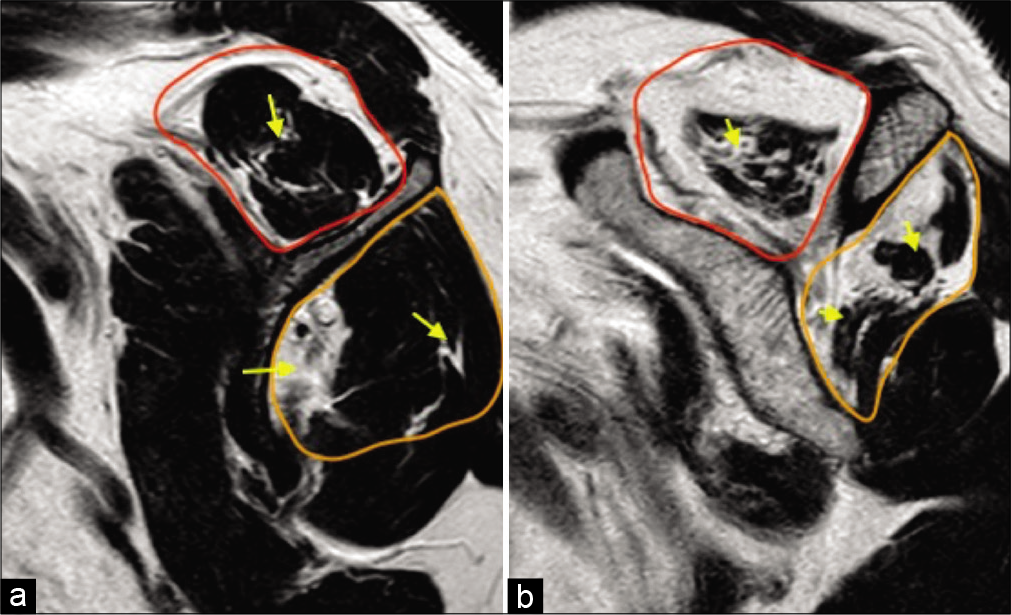
- A 55-year-old female with right shoulder pain and impingement symptoms. (a) T1-weighted sagittal image at the level of scapular neck demonstrating reduced muscle bulk of the supraspinatus (red selection) and the infraspinatus (orange selection), their fatty replacement and fatty infiltration along their myotendinous junctions (yellow arrows) consistent with Grade 1 (early) fatty atrophy and (b) severely reduced muscle bulk (more than 50%), fatty replacement and infiltration along the myotendinous junctions consistent with Grade 3 (advanced) fatty atrophy of the rotator cuff. Shoulder surgeons prefer semi-quantitative Goutallier classification which has prognostic significance.
Arthroplasty options, of increasing invasiveness, include humeral head resurfacing arthroplasty, hemiarthroplasty, anatomical TSA, and RTSA. The type of arthroplasty is typically guided by the indication and the integrity of the deltoid, rotator cuff, glenoid, and humeral head bone stock. For example, anatomical TSA requires a functional rotator cuff and adequate glenoid bone stock, whereas the RTSA only requires an intact deltoid. Hemiarthroplasty is best utilized in patients where an inadequate glenoid bone stock precludes an anatomical TSA, as long as the humeral bone stock is sufficient, and the rotator cuff musculature is intact. Humeral head resurfacing is primarily used in younger patients and is the least invasive arthroplasty option[10] [Figure 13]. Its practice is declining with the emergence of better arthroplasty options. It provides an opportunity for subsequent revision surgery once humeral head resurfacing can no longer achieve optimum function and is usually revised with anatomical or RTSA depending on rotator cuff status.
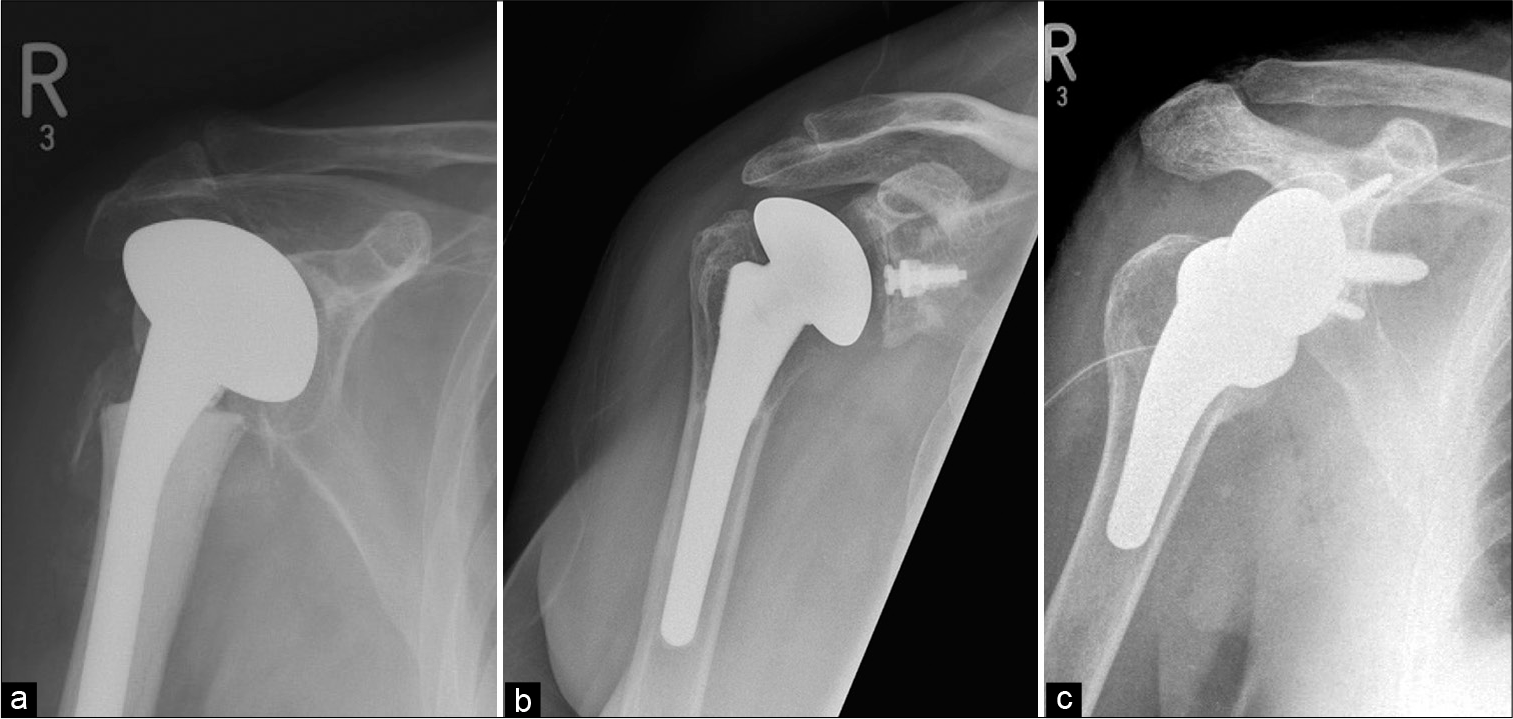
- Different types of shoulder arthroplasties: (a) Hemiarthroplasty – only humeral head is replaced with prosthesis. Please appreciate retention of the native glenoid, (b) total shoulder arthroplasty with non-cemented humeral component and a radiolucent glenoid baseplate with a central peg. It is also known as “anatomical total shoulder replacement” and preferred for patients with functional rotator cuff and (c) reverse total shoulder arthroplasty for cuff-deficient shoulders showing reversed/ inverse relationship of the convex glenosphere and a concave humeral articulating surface at the top of a short-stemmed prosthesis (also seen is a surgical drain).
CONCLUSION
Numerous shoulder arthroplasty options are available to treat a variety of advanced shoulder pathologies. Adequate understanding of shoulder biomechanics and their implications on surgical planning and postsurgical prognosis are critical for the musculoskeletal radiologist. It allows optimum utilization of pre-operative imaging to help surgeons manage the patient’s shoulder ailments in a timely and effective fashion.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Reverse total shoulder arthroplasty: Biomechanics and indications. Curr Rev Musculoskelet Med. 2019;12:542-53.
- [CrossRef] [PubMed] [Google Scholar]
- Hemiarthroplasty for three-and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2012;20:17-27.
- [CrossRef] [Google Scholar]
- Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg Am. 2006;88:1494-500.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome after reconstruction of the proximal humerus for tumor resection: A systematic review. Clin Orthop Relat Res. 2014;472:2245-53.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756-60.
- [CrossRef] [Google Scholar]
- Association between rotator cuff abnormalities and reduced acromiohumeral distance. Am J Roentgenol. 2006;187:376-82.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: A meta-analysis. Am J Roentgenol. 2009;192:1701-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty muscle degeneration in cuff ruptures. Pre-and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78-83.
- [CrossRef] [Google Scholar]
- Shoulder arthroplasty, from indications to complications: What the radiologist needs to know. Radiographics. 2016;36:192-208.
- [CrossRef] [PubMed] [Google Scholar]






