Translate this page into:
Osseous Hydatid Disease – A Series of Cases

*Corresponding author: Rakhee Kumar Paruchuri, Department of Radiodiagnosis, Care Hospital, Hyderabad, Telangana, India. rakheekumar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Paruchuri RK, Kapoor A. Osseous Hydatid Disease – A Series of Cases. Indian J Musculoskelet Radiol 2020;2(2):120-4.
Abstract
Hydatid disease (HD) in the human body is a known entity with liver and lungs being most commonly affected. The disease presentation is well documented. However, primary musculoskeletal HD without any other visceral involvement is uncommon and has a delayed presentation that is often misdiagnosed, especially if limited to the bone. Soft-tissue involvement, if present, can help identify if typical features described are present. We present three interesting cases that presented as diagnostic challenges with varied presentations. In the current scenario of migration and frequent travel, it is important to keep this entity in the vast list of differential diagnoses.
Keywords
Hydatid
Echinococcosis
Musculoskeletal
Magnetic resonance imaging
CT
INTRODUCTION
Hydatid disease (HD) is produced by the larval infestation by Echinococcus granulosus (cystic echinococcosis) and Echinococcus multilocularis (EM) (alveolar echinococcosis). Of the 12 different species identified, only these two are known to affect human beings, with EM being extremely rare and not affecting bone.[1,2] The liver and lung are most commonly involved with typical features well documented in the literature. However, bony involvement is uncommon with atypical features. HD is a known entity in endemic areas, but with the current scenario of migration and frequent travel, it is important to know the imaging features of musculoskeletal HD.
We present three cases of the primary musculoskeletal HD that came to our institute over a period of 5 years.
PATIENT 1
History: A 31-year-old male presented with a history of pain in the right thigh for 3 months with acute pain and inability to walk for 1 day following trivial trauma.
Investigations: AP and lateral radiographs of the thigh [Figure 1] revealed a subtrochanteric fracture with multiple, well defined, medullary, and cortical lytic lesions involving the proximal shaft of the femur with a narrow zone of transition. Endosteal scalloping is noted. Several calcific opacities are seen in adjacent soft-tissue near and distal to the fracture. No discrete soft-tissue mass lesion was noted.
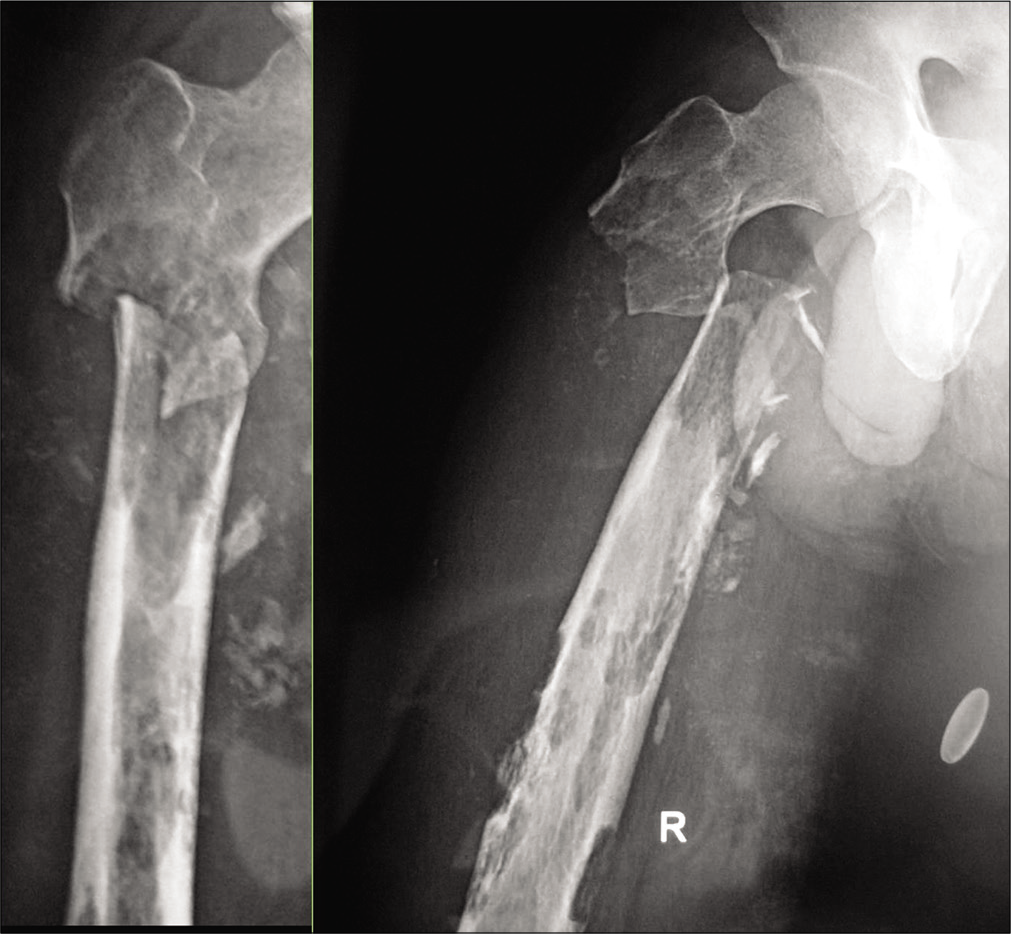
- The patient 1–33 Y/M patient with pain in the right thigh for 3 months and inability to walk for 1 day following trivial trauma: AP and lateral radiographs of the right thigh revealed a subtrochanteric fracture with multiple, well defined intramedullary and cortical lytic lesions of varying sizes in the visualized shaft of the femur with a narrow zone of transition. Cortical thinning and endosteal scalloping are noted. No discrete soft-tissue mass lesion was noted.
Management: Due to financial constraints, no further investigations were performed and the patient was taken up for surgery. Grayish-white material with fluid-filled daughter cysts were observed. Thorough curettage with hypertonic saline packs was performed followed by intramedullary nailing for the fracture. Prophylactic albendazole was started to delay the recurrence.
PATIENT 2
History: A 25 year old female presented with complaints of pain and a slow growing mass at the elbow for 6 months. There was no history of fever, raised local temperature, or redness of the skin.
Investigations: The patient was referred for magnetic resonance imaging (MRI). It revealed [Figure 2] ill-defined, T1W hypointense, and T2W/STIR heterogeneously hyperintense lesions involving the proximal radius and ulna with a cortical breach in the ulna and extension of the cystic lesions into the adjacent muscles. An intermuscular well-defined cystic lesion showing the characteristic hypointense “floating membrane” sign was seen at the level of humerus [Figure 3].
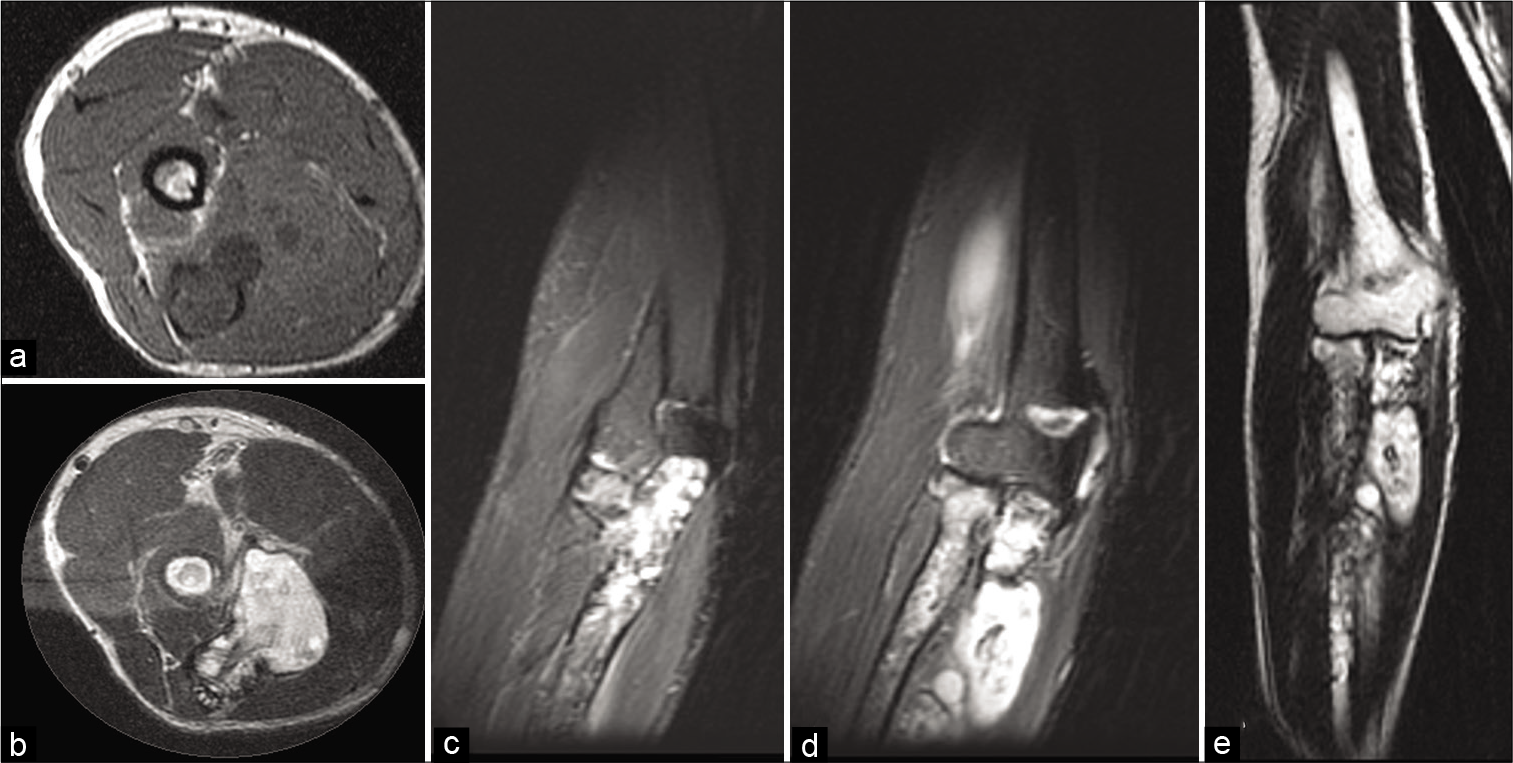
- The patient 2–25 Y/F with gradually increasing swelling at the elbow for 6 months. Image 2 axial T1W (a) T2W (b) coronal STIR (c and d) and T2W (e) magnetic resonance imaging images at the elbow reveal ill-defined, T1 hypointense and heterogeneously T2W hyperintense lesions involving the proximal radius and ulna with a breach in the ulnar cortex and extension of the cystic lesion into the adjacent soft tissues.
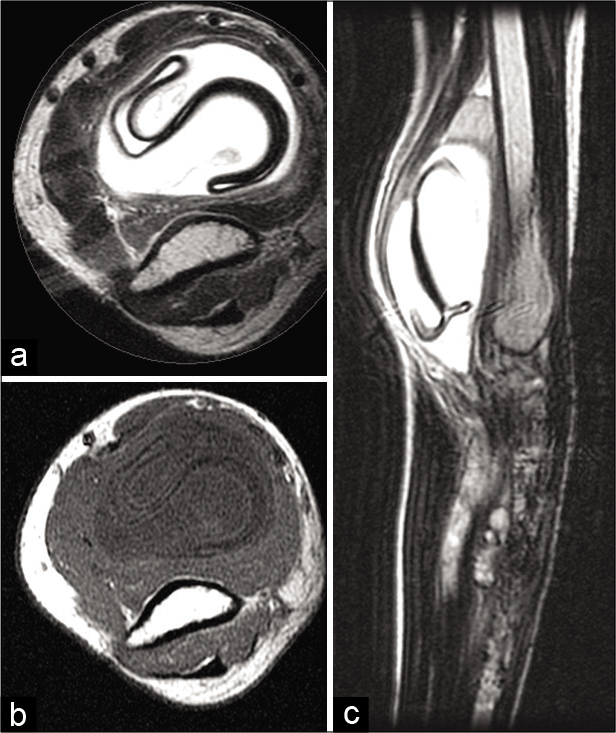
- Same patient as Figure 2. Axial T2W (a), T1W (b), and sagittal T2W (c) images at the humeral level reveal a well-defined cystic lesion showing the characteristic “floating membrane” sign.
USG abdomen and chest radiograph revealed no other lesions.
Management: Surgery with thorough curettage with hypertonic saline packs and care not to spill or tear any of the cysts was performed. The patient was started on prophylactic albendazole.
PATIENT 3
History: A 23-year-old male presented with complaints of backache for 8 months and recent history of chest pain. There was no history of fever, weight loss, or cough. Clinical suspicion of Pott’s spine was entertained.
Investigation: MRI study revealed a [Figure 4] multiloculated, cystic lesion involving D3 and D4 vertebrae with collapse showing extension into the right paravertebral region, spinal canal, and paraspinal muscles. The characteristic pattern of peripherally arranged daughter cysts was identified. On post-gadolinium contrast T1W images, thin peripheral rim of enhancement is seen. The mother cysts are showing isointense (to muscle) signal compared to the hypointense signal of the daughter cysts.
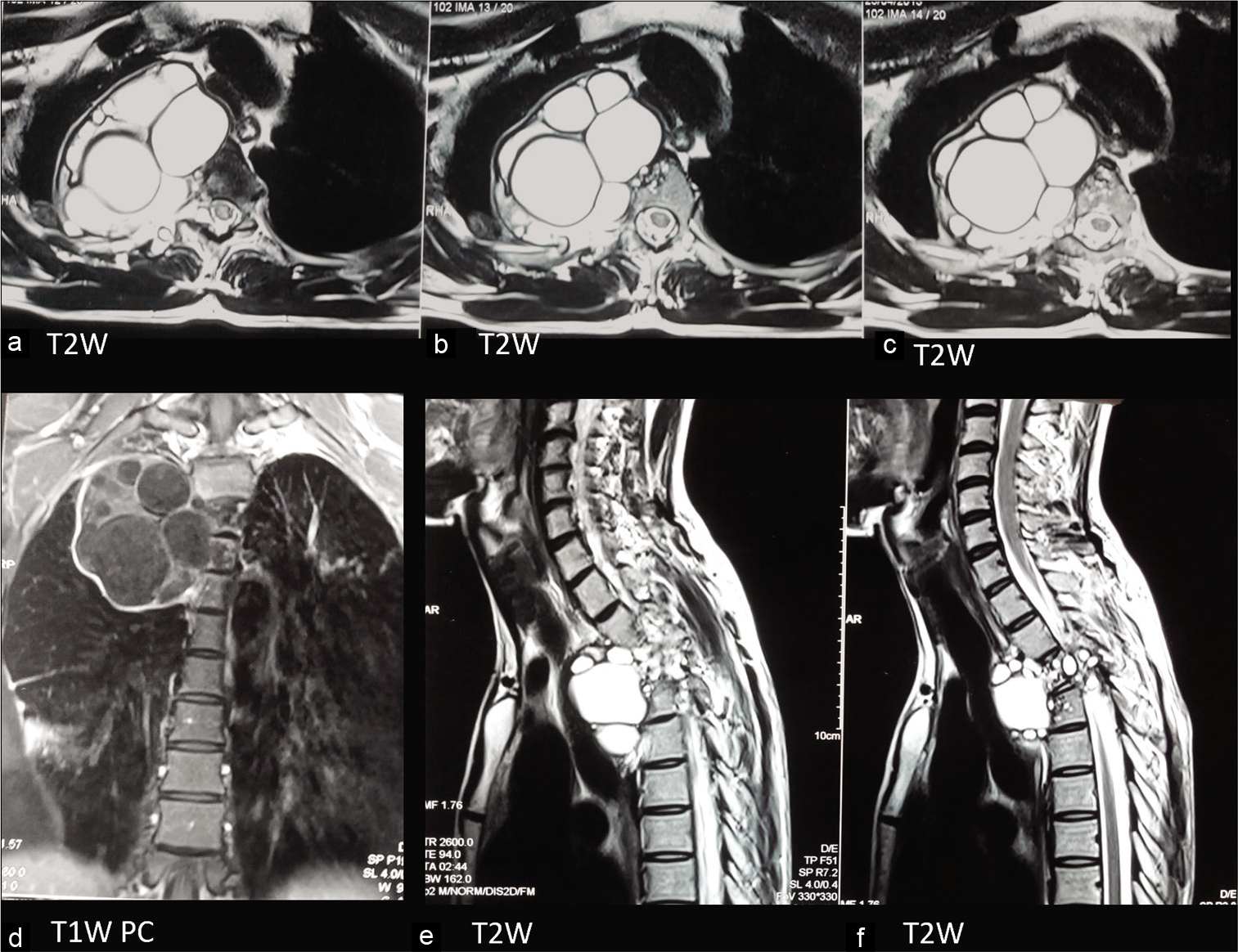
- 23 Y/M with history of back pain since 8 months. Axial T2W (a-c), coronal T1W post-contrast (d), and sagittal T2W (e and f) images through the dorsal spine reveal destructive lesion involving D4 and D5 vertebral bodies with right paravertebral T2W hyperintense multiloculated cystic lesion showing intraspinal extension. T1W PC images (d) show a thin rim of peripheral enhancement. Also note isointense signal of mother cyst compared to the hypointense signal of the daughter cysts.
USG abdomen and chest radiograph revealed no other lesions.
Management: Surgery was performed with pre-operative local injection of 20% cetrimide followed by transthoracic complete cyst excision, removal of necrosed bone with spinal fixation. Prophylactic albendazole was started.
The final histopathological diagnosis for all three patients was osseous hydatidosis, H&E stain showing classical laminated membrane with adjacent areas of inflammation [Figure 5].
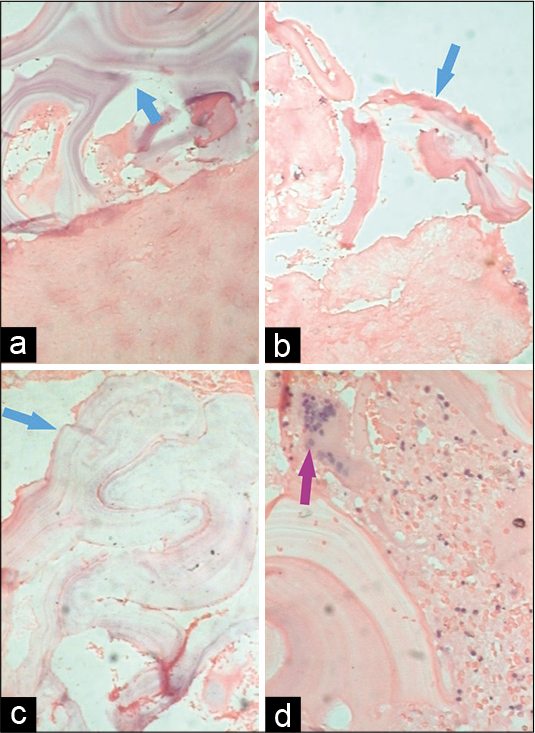
- Histopathology images from Patient 1, H&E stain. Image a, b, c at low power field (×10) reveal hydatid cyst wall consisting of the avascular eosinophilic laminated chitinous membrane (blue arrow). Image d at high power field (×40) shows a foreign body giant cell reaction (purple arrow) with an area of hemorrhage and necrosis.
DISCUSSION
Dogs are the primary host of the echinococcal worm with sheep as the most common intermediate hosts. Humans become intermediate hosts when they come in contact with infected dogs or contaminated plants or soil by direct hand to mouth transfer. Once the eggs are ingested, the escaped embryos are disseminated through venous and lymphatic channels reaching several organs, most commonly the lungs and liver. Osseous involvement has been reported in only 0.5–4% of reported cases, most commonly involving the spine (50–60%), femur, tibia, humerus, skull, and ribs in descending order.[3-5] Bony involvement is almost always due to primary infection, secondary to hematological seeding.[6]
A hydatid cyst (HC) has three layers – the pericyst is the outermost protective layer representing the response of the host to the parasite and is made of modified host cells. The middle laminated membrane is acellular and functions as a passage for nutrients. The inner germinal layer is where the larval stage of the parasite (scoliosis) and the laminated membranes is produced. The middle and inner layers form the true wall of the cyst known as endocyst. The middle layer is also known as the ectocyst. The thickness of these layers depends on their location, with the liver having the thickest layers. In the muscle, they are less developed and are absent in the bone.[1,3]
In the bone, the growth pattern is different compared to other tissues due to the bony resistance of the trabeculae and lack of pericyst formation.[3] The embryos are deposited directly in the bone producing microvesicles by exogenous budding that spread along the path of the least resistance resulting in an irregular branching pattern. The granddaughter vesicles produced invade the bone replacing the medullary tissue. Ischemic necrosis and direct local pressure contribute to bone destruction. In Stage 1, cyst like spaces are produced lined by thin trabeculae giving a multilocular appearance, resembling a “bunch of grapes.” This is identified radiographically as ill-defined multilocular cysts giving “moth-eaten” appearance that is initially epimetaphyseal in a location with a poor zone of transition. No periosteal reaction or proliferation of new bone is noted. Cortical involvement may lead to pathological fractures. Patient 1 was at this stage. In Stage 2, the cysts coalesce resembling a large communicating cyst with a lattice type of trabecular pattern. The HC then produces an inflammatory process around it, resulting in osteitis, causing trabecular bone thickening, bone condensation, cortical bone loss, and hyperostosis. In the third stage, penetration of cortex occurs with the extension of the disease into the soft tissues giving the radiographic appearance of a soft-tissue density mass. Intraosseous cysts generally do not calcify.[7-10]
Imaging on computed tomography (CT) better delineates the lytic lesions as uni or multilocular nature and any soft-tissue extension. If present, characteristic mother and daughter cysts may be visualized giving “rosette” or “spoke wheel” appearance. Song et al. described a round or ovoid cystic lesion with a “double-layered arcuate calcification” as a specific sign for HC compared to any other cyst forming disease.[1]
MRI is considered as the best imaging modality for evaluation of HD as it can show the exact site, extent, and help in the differential diagnosis.[7] The involved bone shows destructive, expansile, and cystic lesions showing heterogeneous intermediate to low-signal intensities on T1W and high-signal intensity on T2W images.[1,3] Song et al. noted that the parent cysts show iso- to hyperintense signal on T1W images which are higher than the signal of daughter cysts. On T2W images, both parent and daughter cysts showed high signal intensities.[1] This was identified in the patient number 3. The multilocular appearance is also a characteristic feature identified on MRI. In a contained rupture, the endocyst can separate from the pericyst resulting in an undulating “floating” membrane, as shown in patient 2.
Spinal HD is the most common site of bony involvement, most commonly involving the thoracic spine. It can be intramedullary, intradural extramedullary, extradural intraspinal, vertebral, or paravertebral in location. Characteristic spinal HD features are lack of osteoporosis and sclerosis, with the disease beginning preferably in the center of the vertebral body and a predilection for involvement of the pedicle. Intervertebral disc involvement is unlikely. Perforation of the cortex and periosteum result may result in extraosseous extension which can be intra or extraspinal. A paraspinal extension may result in the involvement of contiguous ribs (in the thoracic spine).[3,11]
Ultrasound evaluation has a limited role in musculoskeletal HD unless a soft-tissue component is identified, which may show characteristic cystic appearance.
The differential diagnosis of conventional radiology includes several lytic lesions and should include clinical and imaging features to eliminate negative features. Lack of osteosclerosis, location and extension into the soft tissues are clues that may help narrow the diagnosis. For example, GCT is subarticular in location; both fibrous dysplasia and cartilaginous lesions show specific matrix; brown tumors show widespread involvement with generalized osteopenia. In the spine, the involvement of the vertebral endplates should point toward other diagnoses. Skeletal metastases (especially renal or thyroid primary) are also a differential and require further investigations.[6] Once the inflammatory process sets in, osteomyelitis is a close differential. MRI and CT can help to make the final diagnosis, especially if soft-tissue extension is noted, showing the features typical to HC. Clinical and serological correlation also help to achieve the final diagnosis, when imaging findings are non-specific.
SUMMARY
Primary musculoskeletal HD is an uncommon entity that is often misdiagnosed at initial radiological evaluation. Imaging features are non-specific on conventional radiographs and CT evaluation. MRI can identify specific features described to help identify the pathology. In the current scenario of migration and travel, it is important to remember this entity in the differential diagnosis when evaluating bony pathologies.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Combined chemotherapy and surgery for hydatid bone disease. J Bone Joint Surg Br. 1987;69:141-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hydatid disease from head to toe. Radiographics. 2003;23:475-94.
- [CrossRef] [PubMed] [Google Scholar]
- CT characterization of multivesicular hydatid cysts. J Comput Assist Tomogr. 1986;10:428-31.
- [Google Scholar]
- Hydatid disease: Radiologic and pathologic features and complications. Radiographics. 2000;20:795-817.
- [CrossRef] [PubMed] [Google Scholar]
- Radiology of Bone Tumors and Allied Disorders (4th ed). Philadelphia, PA: WB Saunders; 1982. p. :1144-79.
- [Google Scholar]
- The radiology of hydatid disease. AJR Am J Roentgenol. 1985;145:639-48.
- [CrossRef] [PubMed] [Google Scholar]
- Roentgen Diagnosis of Disease of Bone (4th ed). Philadelphia, PA: Williams and Wilkins; 1989. p. :1053-4.
- [Google Scholar]
- Diagnosis and management of hydatid cyst of CNS: Hydatid cysts of skull orbit and spine. Neurosurgery. 2001;11:10-6.
- [CrossRef] [Google Scholar]






