Translate this page into:
Relevance of Scapulothoracic joint assessment for unknown shoulder pain

*Corresponding author: Rajesh Botchu, Department of Musculoskeletal Radiology, Royal Orthopedic Hospital, Birmingham, West Midlands, United Kingdom. drbrajesh@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Vidoni A, Davies M, James S, Botchu R. Relevance of Scapulothoracic joint assessment for unknown shoulder pain. Indian J Musculoskelet Radiol 2022;4:61-9.
Abstract
The scapulothoracic joint (STJ) is one of the four joints forming the shoulder complex with the glenohumeral, acromioclavicular, and sternoclavicular. Abnormal motion of the scapula during shoulder movement is defined as scapular dyskinesia a distinct entity in the orthopedic literature as a source of posterior shoulder pain. Several acute and chronic conditions affecting one or more of the joints of the shoulder complex can result in disorders of the scapular motion. This article aims to provide a comprehensive review of the anatomy and function of the STJ, to describe the MRI features of the internal derangements of this articulation including scapulothoracic bursitis (or snapping scapula syndrome), elastofibroma dorsi, and other tumor and tumor-like lesions.
Keywords
Scapulothoracic joint
Infraserratus bursitis
Supraserratus bursitis
Elastofibroma dorsi
Tumor and tumor-like lesion
INTRODUCTION
The scapulothoracic joint (STJ) is a non-synovial articulation formed by the anterior concave surface of the scapula and the convexity of the chest wall. It has a critical role in the stability and mobility of the shoulder complex. The integrity of the acromioclavicular and sternoclavicular joints provides stability. The synergy between scapular and glenohumeral muscles provides dynamic stability.[1]
The most critical stabilizing and mobilizing muscles of the scapula are the serratus anterior (SA) and the trapezius. The SA contributes to the upward scapular rotation, posterior tipping and external rotation, but, its primary function is to stabilize the scapula against the thoracic wall. The long thoracic nerve provides motor innervation to the SA muscle; neural disorders lead to scapular winging (internal rotation) with the patient being unable to elevate the upper limb.[2,3]
The trapezius cooperates with other muscles in steadying the scapula and controlling its position during shoulder movement and receiving motor innervation from the accessory nerve.[4-6] The presence of two bursae facilitates scapular movements. The infraserratus bursa (ISB) and supraserratus bursa (SSB) bursae are located, respectively, deep and superficial to the SA muscle [Figure 1]. Abnormal scapular mobility or function is defined as scapular dyskinesis (SD) and is the result of shoulder derangements or muscle imbalances.[1,6-9] A constellation of conditions involving the shoulder can result in SD. Rotator cuff tears, glenohumeral instability, labral tears, and impingement syndrome represent common causes of SD. Those are well-described entities with specific radiological and clinical findings whose detailed description is outside the remit of this article. Other common causes of SD are muscle imbalances and, in particular, the presence of increased activity of the SA muscle.[10]

- Anatomy of the scapulothoracic joint: scapula (s), ribs (r), subscapularis muscle (1), serratus anterior muscle (2), supraserratus bursa (*), infraserratus bursa (+).
This article aims to review the internal derangements of the STJ responsible for SD describing the relevant MRI findings.
TECHNICAL CHALLENGES: IMAGING OF THE STJ
Radiographs are usually the first line of examination and may be useful to identify bony abnormalities with the Y view particularly effective in assessing for bony incongruences. Ultrasound has a minimal diagnostic role as the ultrasonic waves cannot penetrate the bone and visualize the joint interface. However, it can be useful in guiding injections in the presence of bursal effusion.[11]
Cross-sectional imaging techniques are the modality of choice for a comprehensive depiction of the STJ. First, CT allows isotropic acquisition with the possibility of three-dimensional reconstruction. It is particularly useful not only for the characterization of bone abnormalities but also for the visualization of soft tissue masses, mineralization and the presence of fat. In case bilateral masses are demonstrated, those are diagnostic for elastofibroma dorsi (ED).[12]
MRI can depict all the components of the STJ [Figure 2]. The acquisition protocol should include images obtained along at least two acquisition planes: Axial and coronal (oriented along the long axis of the scapula) [Figure 3] and for each plane a morphologic sequence (PD-weighted or T1-weighted) and a fluid-sensitive sequence (fat-saturated PD/ T2-weighted or STIR).[13]

- MRI Anatomy on a T1-weighted axial sequence: Scapula (S), ribs (dashed line), subscapularis muscle (solid line) and serratus anterior muscle (dotted line); an expected position of the supraserratus bursa (arrowhead), infraserratus bursa (arrow) are also indicated.

- Acquisition planes: Axial (a) and coronal (b).
The use of BLADE (rotating blade-like k-space covering) sequences prevents motion truncation and flow artifacts. In these sequences, the rectilinear k-space sampling is replaced by a rotating partially overlapping fashion (blades) to minimize the deterioration of images secondary to physiologic body movement.[14]
Imaging has a significant impact on clinical management and, in case surgery is necessary MRI has a pivotal role in the preoperative planning.[15,16]
CONDITIONS AFFECTING THE STJ
Scapulothoracic bursitis (STB)
STB, snapping scapula syndrome, and scapulothoracic crepitus are generic terms used to identify a pathologic entity first described by Boinet in 1867[15,16] and characterized by inflammatory change and effusion involving either the ISB or the SSB.[16-21] STB is a relatively uncommon entity, but it is among the most frequent disorders involving the STJ. Bursitis is suspected in the presence of an abnormal relationship between the anterior surface of the scapula and the chest wall resulting in scapular snapping. Periscapular tenderness, pain with increasing shoulder activity, and palpable crepitus characterize the symptomatology.[16]
The most common causes of STB are bony incongruences between the scapula and the chest wall that can be congenital [Figure 4], acquired (malunion of a rib or a scapular fracture), neoplastic (osteochondroma Figure 5) and secondary to chronic overuse in particular in overhead athletes (throwing, swimming, and tennis).[16,19,22]

- Axial T1-weighted (a), axial T2 (b), FS coronal T1-weighted (c), coronal STIR (d). Supraserratus bursitis (dashed line) secondary to an osteochondroma arising from the anterior surface of the scapula (arrow).

- T1-weighted axial (a), T2-weighted fat-saturated axial (b) supraserratus and infraserratus bursitis. The small arrow indicates the communication between the supraserratus (dot) and the infraserratus bursa (square) the big arrow indicates the serratus anterior muscle.
In the acute phase, bursitis appears on MRI as a fluid lesion (hyperintense on fluid-sensitive sequences and hypointense on T1-weighted sequences) surrounded by a thin hypointense rim. In the subacute phase, multiple frond-like structures hypointense in all the pulse sequences and arising from the peripheral walls can be seen reflecting the prolonged synovitis. The chronic phase is characterized by the presence of multiple, thick internal septa which are hypointense in all the pulse sequences[21,23] [Figures 4 and 5].
The initial treatment of STB should be non-operative and consist of rest, nonsteroidal anti-inflammatory drugs, activity modification, and shoulder rehabilitation (massage, ultrasound, and iontophoresis). Sterile US-guided injections of corticosteroids and local anesthetics can also be performed and usually have both a diagnostic and therapeutic value.[16] The use of computed tomography (CT) guidance can minimize the potential risk of pneumothorax.[11] Surgery represents the option of choice in case of bursitis secondary to osteochondroma.[22]
Elastofibroma Dorsi (ED)
ED is a slow-growing, nonencapsulated, fibroelastic pseudotumor considered to be reactive to the repetitive mechanical friction between the scapula and the chest wall. ED has a prevalence of 2% in the population over 60 years old with a female predilection.[24] It usually affects the STJ and is located deep in the SA muscle, at the level of the distal aspect of the scapula. In 60% of cases, it is present bilaterally. Approximately 50% of the patients experience only moderate discomfort characterized by clicking with the movement of the arm. ED is usually an incidental finding, and, there is a lack of consensus in the description of specific clinical symptomatology.[25,26]
On CT it appears as an irregular soft-tissue mass located in the inferior subscapular region, the presence of thin strikes of tissue with a density equal to fat is diagnostic.[12,24]
The correlation between histology and MRI appearances is crucial for the diagnosis of ED. This lesion has two different components: A prominent fibrous portion containing collagen bundles and elastic fibers that are hypointense on both T1-weighted and T2-weighted sequences and sparse internal fatty streaks containing mature adipose cells which show high signal intensity on T2-weighted and T1-weighted sequences with homogeneous suppression on fluid-sensitive sequences[24] [Figure 6]. The presence of a lesion with these imaging features located within the scapular region is usually diagnostic.

- T1-weighted axial (a) and (b) Bilateral Elastofibroma Dorsi (dashed line) appearance of the main components of the lesion, fibrous tissue (arrowhead) with interposed fatty “streaks” (arrow).
Given these considerations and the benign nature of the lesion, surgical excision is not necessary and should be reserved for symptomatic patients.[27,28] The ED is usually firmly adherent to the SA muscle and the rib cage. Hence, surgery is affected by a high incidence of complications, including the development of a seroma and hematoma.[29]
Bursitis and ED are the most common lesions affecting the STJ. These are benign lesions that are usually managed conservatively. However, many different pathologies with specific MRI features can also occur at this level. These include benign and malignant entities that present specific clinical management. Misinterpretation of the findings can potentially result in a late diagnosis with potential harm to the patient. We provide a comprehensive description of these lesions with an emphasis on the specific imaging features.
Giant cell tumor (GCT)
GCT of bone is a rare benign, locally aggressive and rarely metastatic tumor of bone. It affects the scapula in <1%. Patients usually are in the age group between the 2nd and the 5th decade of life with a slight female predilection. Symptoms of scapular GCT are related to the consequent incongruence of the STJ that affects the biomechanics and poses a significant challenge for the clinician. Surgical resection is the therapy of choice. Medical therapy with denosumab is the treatment of choice for incompletely excised lesions.[30,31]
CT may present as an eccentric enlargement of the scapula with a soft-tissue mass[31] [Figure 7].

- T1-weighted axial (a) T2-weighted fat-saturated axial (b), T1-weighted coronal (c) and STIR coronal (d). GCT of the scapula involving the ST joint causing incongruence of the scapulothoracic joint and atypical posterior shoulder pain.
On MRI it is characterized by a well-defined non-sclerotic margin. GCT shows intermediate-low signal intensity in T1W sequences and high signal intensity in T2W/fluid-sensitive sequences.
Lipoma
Lipoma represents the most common soft tissue neoplasm (approximately 50% of all soft-tissue tumors) typically it has benign behavior and affects the age group between the 3rd and the 6th decades with no sex predilection. Lipomas are divided based on their location, either superficial or deep: The majority of them are identified within the subcutaneous tissues. The patient usually presents with a slow-growing painless lump.[32] Deep lipomas are usually incidental findings and are asymptomatic. However, when lipoma involves the STJ in (22% of the cases), it results in pain and fatigability of the shoulder and asymmetric scapular position.[32,33] Malignant lipomatous tumors are unusual, and only the presence of masses over 10 cm, areas of fat necrosis and inflammation, the presence of thick septa and calcifications should raise the suspicion for atypical behavior.[34] Excision represents the option of choice for symptomatic lipomas.
Usually, on MRI, it is easy to give a specific diagnosis in the presence of a lesion that shows signal intensity equal to adipose tissue (hyperintense in T1W and T2W sequences, homogeneous suppression on fluid-sensitive sequences). Information about the presence of non-lipomatous areas, such as septa, calcification, and solid components should be included in the report[32,34] [Figure 8].

- T1-weighted axial (a), T2-weighted fat-saturated axial (b), T1-weighted coronal (c), STIR coronal (d). Intramuscular ALT involving the subscapularis. The lesion is bigger than 10 cm and on fluid-sensitive sequences with areas of increased signal intensity (dashed lines in b and d). At the histologic examination, the benign nature was confirmed.
Vascular malformation (VM)
VMs usually involve the STJ in about 20% of the cases.[35] The presence of a VM affecting the STJ can alter the biomechanics and has a potential for pain. Embolization represents the option of choice.[36]
On histology is characterized by simple VMs with slow flow and evidence of abnormal venous network and large dysplastic vascular channels and the presence of fat between the vessels. On MRI, the lesion demonstrates high signal intensity on T2W and fluid-sensitive sequences and low signal intensity on T1W sequences. Usually, the abnormal, dysplastic vessels are easily identifiable and should point toward the diagnosis of VM. The detection of small voids of signal on all pulse sequences representing small foci of calcification (phleboliths) confirms the diagnosis [Figure 9]. Of particular importance is the fluid-sensitive sequences to define the topographic extent of the malformation.[36]

- T1-weighted axial (a) T2-weighted fat-saturated axial (b), T1-weighted SE coronal (c) and STIR coronal (d). Venous malformation in the scapulothoracic joint. A large mass containing large dysplastic vascular channels with some interposed tiny foci hypointense on all pulse sequences (phleboliths arrows in a and b) is demonstrated.
Peripheral nerve sheath tumors (PNST)
The term PNST usually is referred to schwannomas and neuromas, whose imaging appearances are mostly overlapping. They affect patients mainly in the 2nd and 3rd decades, with no sex predilection. Involvement of the brachial plexus is sporadic, and only a few cases of STJ involvement were reported in the literature.[37,38] Usually, they are solitary, presenting slow growth and small dimensions (lesions larger than 5 cm are rare). The presence of multiple lesions should raise the possibility of neurofibromatosis. Neurological symptoms are usually exacerbated by palpation. A benign PNST affecting the STJ can be a cause of neuropathic shoulder pain.
On MRI the PNST appears as a mass with low signal intensity on T1W sequences. However, with T2W/fluid-sensitive sequences the Schwannoma demonstrates high signal intensity with a central area of low signal intensity (target sign); with T1W sequences a fine rim of fat is usually seen around the lesion (split-fat sign). For large lesions, episodes of intralesional hemorrhage and cystic change are considered normal[39] [Figure 10].

- T1-weighted axial (a) T2-weighted fat-saturated axial (b). Peripheral nerve sheath tumors (Schwannoma) involving the scapulothoracic joint. Low T1W signal intensity lesion in (a) with high signal intensity on fluid-sensitive sequences (b). A central area of low signal intensity (arrow in b) is known as the “target sign.”
Intramuscular myxoma
The intramuscular myxoma has an abundant myxoid matrix with few spindle-shaped stromal cells. Approximately 17% of the myxomas are intramuscular and can involve any muscle.[40]
On MRI it appears as an ovoid-like lesion with a fluid signal appearance: low signal on T1w sequences and high signal intensity on T2W/fluid-sensitive sequences. There are also typical findings that can improve the diagnostic accuracy of MRI. The first feature is the so-called “fat rind sign” which is a small collection of adipose tissue visible just adjacent to the myxoma and easy to detect on T1W sequences (fat suppression is useful to confirm). The second sign is an ill-defined area of mild edema in the adjacent muscle (increased signal visible on fluid-sensitive sequences)[41] [Figure 11].

- Axial T1-weighted (a), axial T2-weighted fat-saturated (b and c). Intramuscular myxoma of the subscapularis, typical MRI features: low T1 signal intensity (dotted line in a) and fat rind sign (arrow in a). Mild edema of the muscle fibers in contact is considered a typical feature (arrow in c).
Fibromatosis
Fibromatosis (according to the WHO classification Desmoid-Type Fibromatosis) is a benign fibroblastic tumor capable of locally aggressive potential and with a high recurrence rate after surgical excision. It occurs in patients usually between the 2nd and 4th decades, and a female predilection has been described among the younger patients. Shoulder/upper arm and chest wall are involved, respectively, in 28% and 17% of the cases.[42,43] Three different stages define the clinical course of this pathology. The first (early) stage is characterized by the prominence of the cellular (fibroblastic) component. In the second stage, a progressive increase of the collagen component represents the main feature. Whereas, in the third stage, the collagen is the main component and the fibroblasts are scarce or absent.
The MRI appearance of fibromatosis is very variable due to the modifications of the composition typical of the history of this pathology. Therefore, lesions with low collagen content and a high cellular component will show high signal intensity on T2W/fluid-sensitive sequences (myxoid degeneration also contributes to the T2 signal elevation) [Figure 12] whereas lesions with high collagen content and low cellular component show low signal intensity or both in T1W sequences and T2W/fluid-sensitive sequences[43] [Figure 13].

- Axial T1-weighted (a) and axial T2-weighted fat-saturated (b) desmoid-type fibromatosis in the scapulothoracic joint the presence of high signal intensity on T2-weighted sequences indicates a lesion with high cellular content (early stage).

- Axial T1-weighted (a) and axial T2-weighted fat-saturated (b) desmoid-type fibromatosis in the scapulothoracic joint arrows indicate the presence of high signal intensity on T2-weighted sequences indicates a fibrous lesion with poor cellular content (late-stage).
Soft-tissue sarcoma
Soft-tissue sarcomas are very rare, representing about 1% of all malignant tumors with the scapular area representing the 4th most commonly involved area after hip, knee and ankle. Soft-tissue malignant tumors are classified histologically from the adult tissue they resemble (muscle, fat, fibrous tissue, vascular structures, and peripheral nervous structures). In 5–15% of the cases, sarcomas present poorly differentiated cells, and it is not possible to reach a definitive histologic diagnosis. MRI features of aggressive soft-tissue sarcomas are often not specific. Usually, they show heterogeneous morphology characterized by signal intensity mainly similar to the skeletal muscle in T1, high signal intensity in T2 with areas of necrosis, and hemorrhage characterized by fluid-fluid levels[44,45] [Figure 14].

- Axial T1-weighted (a) T2-weighted fat-saturated axial (b), coronal T1-weighted (c) and STIR coronal (d) soft tissue Ewing’s sarcoma, a large soft tissue mass is noted in the ST articulation, the presence of some hemorrhagic areas with fluid-blood level reflects the aggressive biological behavior (arrow a and b).
CONCLUSION
STJ is one of the joints of the shoulder complex. A spectrum of pathologies can involve the STJ and awareness of this is essential to decrease morbidity and mortality. Our articles stress the importance of reviewing the STJ while reporting a shoulder (MRI, CT, X-ray, or ultrasound) [Flow Chart 1].
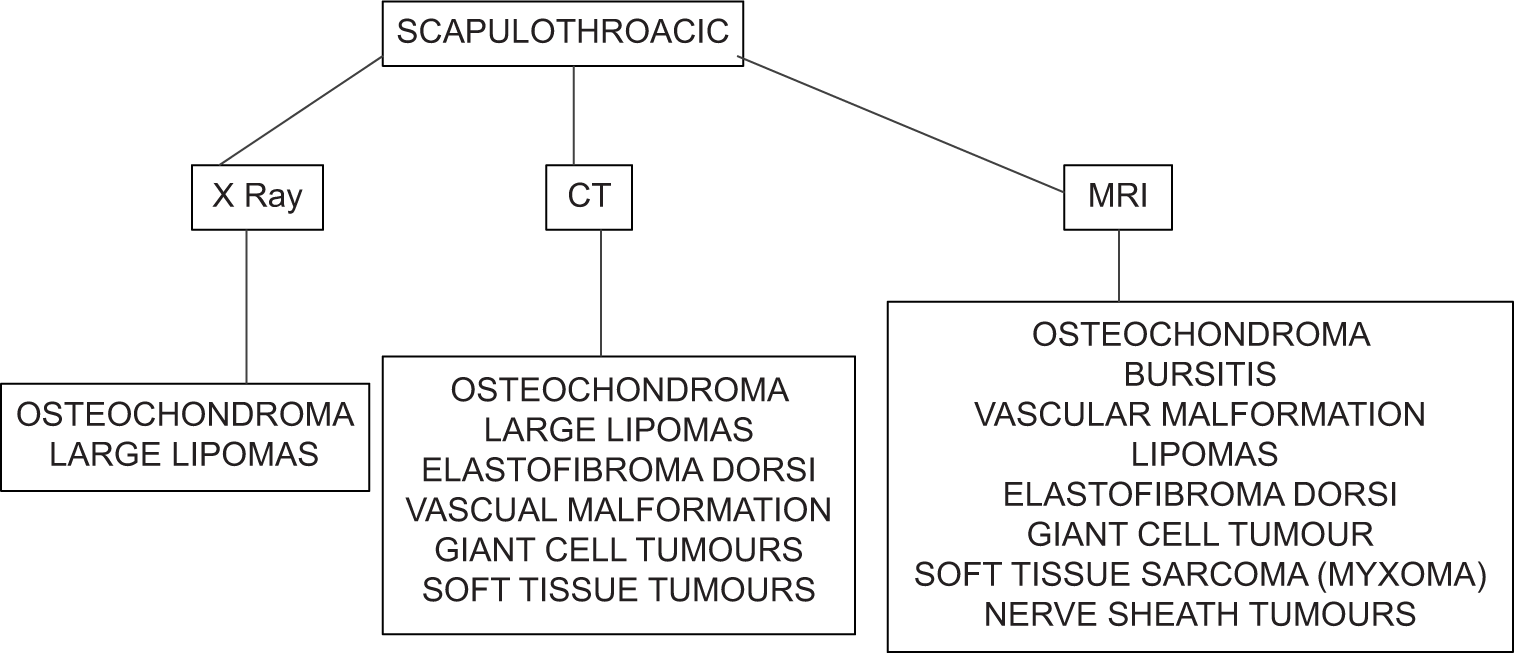
- Flow chart showing the use of different modalities for diagnosis of scapulothoracic joint pathologies.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Rajesh Botchu is on the Advisory Board of the journal.
References
- Current concepts: Scapular dyskinesis. Br J Sports Med. 2010;44:300-5.
- [CrossRef] [PubMed] [Google Scholar]
- An anatomic study of structure and innervation of the serratus anterior muscle. Surg Radiol Anat. 2012;34:921-8.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of long thoracic nerve on high-resolution 3T MRI. Clin Imaging. 2020;64:97-102.
- [CrossRef] [PubMed] [Google Scholar]
- The innervation of the trapezius muscle. An electrophysiological study. J Neurol. 1974;207:183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Topographical and functional anatomy of trapezius muscle innervation by spinal accessory nerve and C2 to C4 nerves of cervical plexus. Surg Radiol Anat. 2016;38:917-22.
- [CrossRef] [PubMed] [Google Scholar]
- Relative balance of serratus anterior and upper trapezius muscle activity during push-up exercises. Am J Sports Med. 2004;32:484-93.
- [CrossRef] [PubMed] [Google Scholar]
- Scapular dyskinesis: The surgeon's perspective. Shoulder Elbow. 2015;7:289-97.
- [CrossRef] [PubMed] [Google Scholar]
- Shoulder kinematics and spatial pattern of trapezius electromyographic activity in real and virtual environments. PLoS One. 2015;10:1-18.
- [CrossRef] [PubMed] [Google Scholar]
- Scapulothoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability. J. Electromyogr Kinesiol. 2014;24:277-84.
- [CrossRef] [PubMed] [Google Scholar]
- Scapular dyskinesis: From basic science to ultimate treatment. Int J Environ Res Public Health. 2020;17:7-9.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging-guided treatment of scapulothoracic bursitis. HSS J. 2007;3:213-5.
- [CrossRef] [PubMed] [Google Scholar]
- Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. Am J Roentgenol. 1992;159:575-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics. 2014;34:217-33.
- [CrossRef] [PubMed] [Google Scholar]
- Elimination of motion and pulsation artifacts using BLADE sequences in shoulder MR imaging. Skeletal Radiol. 2015;44:1619-26.
- [CrossRef] [PubMed] [Google Scholar]
- Interscapulothoracic resection of tumors of the shoulder with a note on reconstruction. Bone Joint J. 2014;96B:684-90.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical management of scapulothoracic bursitis and the snapping scapula. Sports Health. 2010;2:147-55.
- [CrossRef] [PubMed] [Google Scholar]
- The superomedial bare area of the costal scapula surface: A possible cause of snapping scapula syndrome. Surg Radiol Anat. 2013;35:95-8.
- [CrossRef] [PubMed] [Google Scholar]
- Symptomatic bursa formation with osteochondromas. Am J Roentgenol. 1979;133:895-8.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondroma of the scapula associated with winging and large bursa formation. Med Princ Pract. 2006;15:387-90.
- [CrossRef] [PubMed] [Google Scholar]
- Scapulothoracic bursitis of the chest wall. J Ultrasound Med. 2005;24:1437-40.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopically assisted resection of a scapular osteochondroma causing snapping scapula syndrome. World J Surg Oncol. 2007;5:37.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and imaging features of distended scapulothoracic bursitis: Spontaneously regressed pseudotumoral lesion. J Comput Assist Tomogr. 2004;28:223-8.
- [CrossRef] [PubMed] [Google Scholar]
- Best cases from the AFIP: Elastofibroma Dorsi. Radiographics. 2006;26:1873-6.
- [CrossRef] [PubMed] [Google Scholar]
- Elastofibroma dorsi: An uncommon benign pseudotumor. Sarcoma. 2008;2008:756565.
- [CrossRef] [PubMed] [Google Scholar]
- MR and CT Appearance with radiologic-pathologic correlation elastofibroma. AJR Am J Roentgenol. 1992;159:575-9.
- [CrossRef] [PubMed] [Google Scholar]
- Surgery for elastofibroma dorsi: Optimizing the management of a benign tumor-an analysis of 70 cases. J Thorac Dis. 2020;12:1884-94.
- [CrossRef] [PubMed] [Google Scholar]
- Elastofibroma dorsi: A rare connective tissue tumor. Cureus. 2020;12:e6874.
- [CrossRef] [Google Scholar]
- Elastofibroma dorsi: Surgical indications and complications of a rare soft tissue tumor. Mol Clin Oncol. 2014;2:421-4.
- [CrossRef] [PubMed] [Google Scholar]
- Giant-cell tumor of bone: Treatment options and role of denosumab. Biol Targets Ther. 2015;9:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of bone: Review, mimics, and new developments in treatment. Radiographics. 2013;33:197-211.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP: Benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433-66.
- [CrossRef] [PubMed] [Google Scholar]
- MRI characteristics of lipoma and atypical lipomatous tumor/well-differentiated liposarcoma: Retrospective comparison with histology and MDM2 gene amplification. Skeletal Radiol. 2013;42:635-47.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of fatty tumors: Distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224:99-104.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous malformations of the extremities: MR imaging. Radiology. 1986;151:475-9.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics 201;. ;31:1321-40.
- [CrossRef] [PubMed] [Google Scholar]
- Schwannoma of the brachial plexus; report of two cases involving the C7 root. J Brachial Plex Peripher Nerve Inj. 2013;8:12.
- [CrossRef] [PubMed] [Google Scholar]
- Schwannoma of the suprascapular nerve: A case report. J Shoulder Elbow Surg. 2017;15:127-9.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: Radiologic-pathologic correlation. Radiographics. 1999;19:1253-80.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of soft-tissue myxoma with emphasis on CT and MR and comparison of radiologic and pathologic findings. Radiology. 2002;225:215-24.
- [CrossRef] [PubMed] [Google Scholar]
- Intramuscular myxoma: Characteristic MR imaging features. Am J Roentgenol. 2002;178:1255-9.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP: Musculoskeletal fibromatoses: Radiologic-pathologic correlation. Radiographics. 2009;29:2143-73.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of musculoskeletal fibromatosis. Radiographics. 2001;21:585-600.
- [CrossRef] [PubMed] [Google Scholar]
- Radiologic evaluation of soft-tissue masses. Am J Roentgenol. 2000;175:575-87.
- [CrossRef] [PubMed] [Google Scholar]
- From the radiologic pathology archives: Ewing sarcoma family of tumors: Radiologicpathologic correlation. Radiographics. 2013;33:803-31.
- [CrossRef] [PubMed] [Google Scholar]






