Translate this page into:
Revisiting ultrasound assessment of median nerve in carpal tunnel syndrome: A review

*Corresponding author: Vaishali Upadhyaya, Department of Radiology, Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow, Uttar Pradesh, India vshali77@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Upadhyaya V, Choudur HN. Revisiting ultrasound assessment of median nerve in carpal tunnel syndrome: A review. Indian J Musculoskelet Radiol. 2024;6:88-94. doi: 10.25259/IJMSR_7_2024
Abstract
Carpal tunnel syndrome (CTS), which occurs due to compression of the median nerve as it traverses the carpal tunnel at the level of the wrist joint, is the most common entrapment neuropathy. Conventionally, clinical evaluation and electrodiagnostic tests such as nerve conduction velocity and electromyography have been the mainstay of diagnosis in patients with clinically suspected CTS. In recent times, ultrasound (US) has become increasingly popular for diagnosing CTS. However, despite its widespread popularity, the criteria used for diagnosis vary widely. This paper aims to discuss multiple studies which evaluate the role of US in CTS and try to clarify which US criteria can be used with ease and accuracy in daily clinical practice.
Keywords
Median nerve
Ultrasound
Carpal tunnel syndrome
Entrapment
Neuropathy
INTRODUCTION
Compression of the median nerve as it passes through the carpal tunnel at the level of the wrist joint results in carpal tunnel syndrome (CTS), which is the most common entrapment neuropathy. In the United States, its incidence and prevalence are 1–3 persons per thousand population annually and 50 per thousand population, respectively.[1] Nerve compression lowers the patient’s quality of life by causing persistent pain and functional impairment. The aim of pertinent investigations should be to timely diagnose the condition so that it can be managed appropriately, and permanent nerve damage can be prevented.
When evaluating patients with clinical symptoms suggestive of CTS, the traditional approach has been to use electrodiagnostic procedures such as nerve conduction velocity and electromyography (EMG) in addition to clinical examination. However, high cost, long examination time, and patient discomfort are some of the problems associated with these tests. In addition, they are unable to distinguish between the numerous causes of CTS and can yield both false-negative and false-positive results. In recent times, ultrasound (US) has gained popularity for imaging of CTS due to its easy availability, decreased cost, and lack of invasiveness or adverse effects. It can be done in a short span of time and enables high-resolution and dynamic imaging of the median nerve in the carpal tunnel.[2-6] Magnetic resonance imaging also has high accuracy in the diagnosis of CTS due to its superior soft-tissue resolution and multiplanar imaging capability. However, it is expensive, less widely available, has a longer examination time, and does not allow dynamic imaging. Therefore, due to its advantages, the US is the preferred imaging modality when evaluating individuals who may have CTS.[7,8]
Although the use of high-resolution US has slowly gained widespread popularity for the diagnosis of CTS; yet, the criteria used for diagnosis vary widely. Nerve compression occurs within the carpal tunnel, and the nerve is enlarged proximally, increasing the cross-section area (CSA) of the nerve in affected individuals. US diagnosis of CTS, therefore, relies on the measurement of the CSA of the median nerve. However, there is wide variability among different studies as to where the CSA should be measured and whether any additional criteria should be used to improve the diagnostic accuracy of the US.[9,10] In this paper, the authors discuss multiple studies that evaluate the role of the US in CTS and try to clarify which US criteria can be used with ease and accuracy in daily clinical practice.
PATHOPHYSIOLOGY OF CTS
The tendons of the flexor digitorum profundus, flexor digitorum superficialis, flexor pollicis longus, and median nerve pass through the carpal tunnel, which is a fibro-osseous tunnel, whose boundaries include the flexor retinaculum and the carpal bones. Any disease process causing a reduction in the tunnel size or enlargement of the structures within the tunnel leads to median nerve compression, which can manifest clinically as pain, tingling, numbness, and paresthesias in the nerve distribution.[11]
Although nerve entrapment is commonly idiopathic, other conditions identified as causative factors include trauma, osteoarthritis, mass lesions including neurogenic tumors, ganglion cysts or lipomas, inflammatory or infective tenosynovitis, accessory muscles, and systemic diseases including hypothyroidism and diabetes.[11-13] When nerve compression occurs, it causes local circulatory disturbances in the nerve, leading to venous congestion and proximal neural edema. This further compromises both arterial and venous blood flow. Subsequently, fibrosis occurs with nerve entrapment, resulting in demyelination and axonal damage.[14]
SONOGRAPHIC TECHNIQUE AND NORMAL APPEARANCE
A linear-array transducer with a frequency of 5–17 MHz is used to carry out the US of the median nerve. The patient is seated with the forearm supine in front of the radiologist. The hand and wrist are stabilized on a flat surface, and the fingers are partially flexed. In the forearm and wrist region, the nerve is assessed in both transverse and longitudinal planes.[5,9]
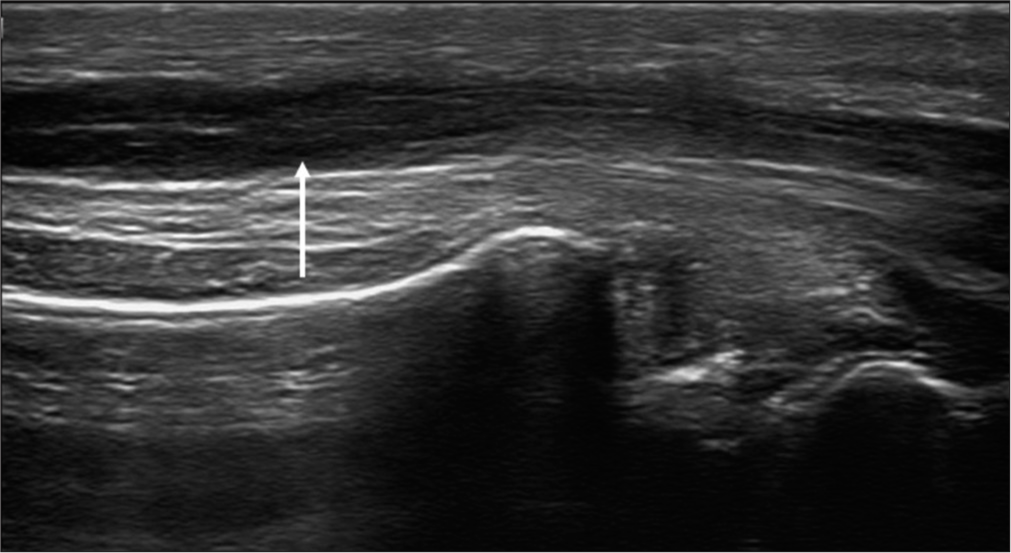
In the short axis plane, a honeycomb appearance of the normal median nerve is seen as the neural fascicles appear hypoechoic and the surrounding connective tissue appears echogenic. In the long-axis plane, the nerve demonstrates alternating linear tubular hypoechoic and echogenic bands from the neural fascicles and intervening connective tissue [Figure 1]. The connective tissue layers include the endoneurium, perineurium, and epineurium, which cover the axon, fascicle, and nerve, respectively. Although the endoneurium is very thin and not visualized in the US, the perineurium and epineurium are thick enough to be seen and appear hyperechoic in the US.[15] Nerves can be differentiated from adjacent tendons which appear brighter with a fibrillar appearance.
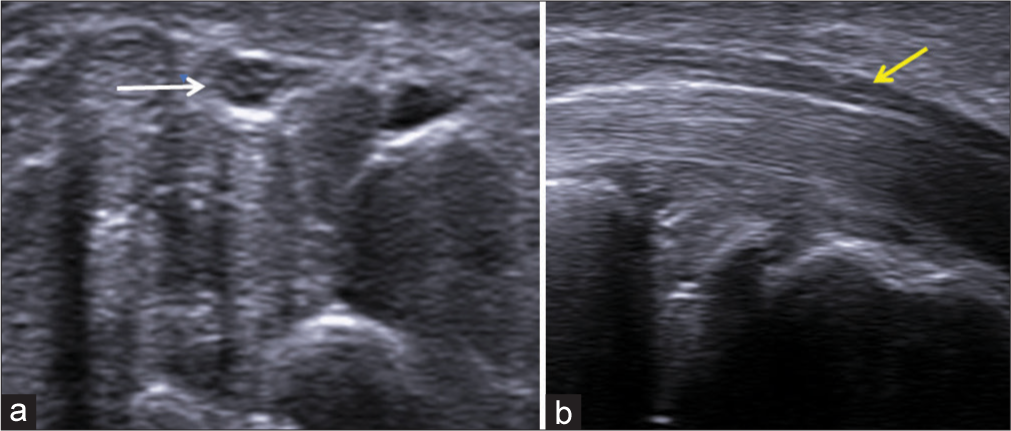
- (a) Transverse axis and (b) longitudinal axis ultrasound images of the median nerve showing the honeycomb appearance of the nerve (white arrow) and alternating hypoechoic and hyperechoic bands (yellow arrow), respectively.
Positioning the US beam in a direction perpendicular to the nerve enables optimal nerve visualization in the short axis.[12,16,17] The CSA of the nerve is measured along the inner margin of the epineurium [Figures 2 and 3]. The mean CSA of the median nerve in healthy individuals varies from 6.1 to 10.4 mm2.[9]
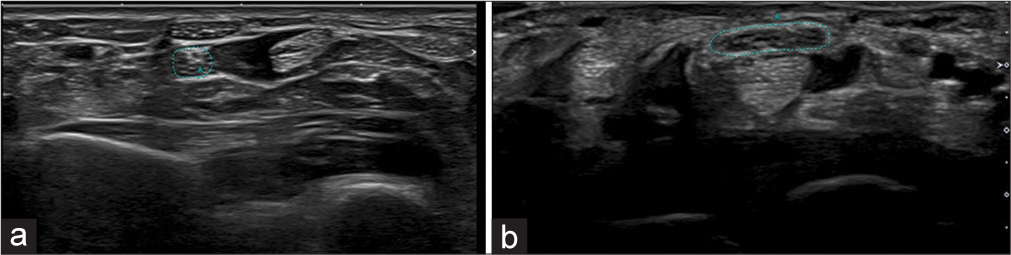
- (a) Transverse axis ultrasound images at the level of the pronator quadratus (Green dots) and (b) the level of the carpal tunnel showing measurement of the cross-section area of the median nerve (Green dots).
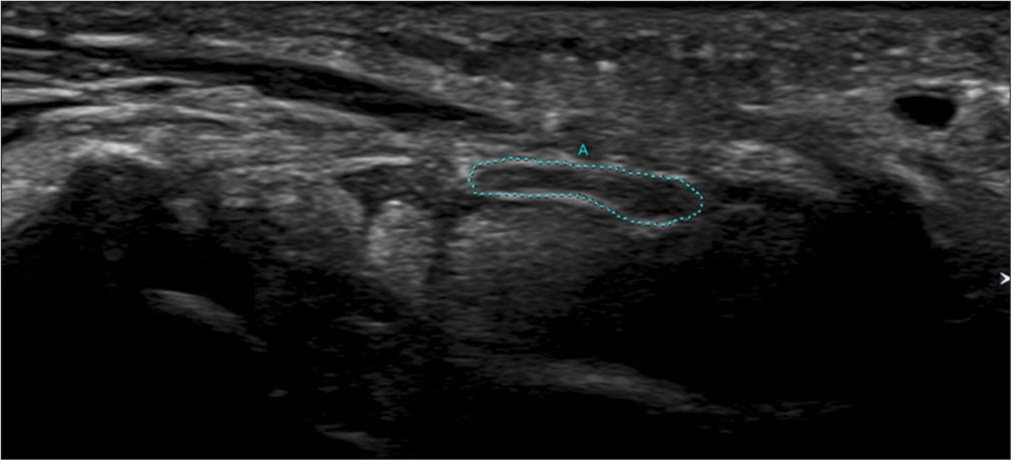
- Transverse axis ultrasound image showing measurement of the cross section area of the median nerve (A) distal to the outlet of the carpal tunnel (Green dots).
SONOGRAPHIC ANALYSIS OF THE MEDIAN NERVE IN CTS
US findings in CTS have been studied extensively, and there are a large number of criteria that have been used by different researchers to enable the diagnosis of CTS with high accuracy. However, there is wide variation in these studies regarding the level of measurement of the CSA of the nerve and its cutoff value. Besides CSA, the role of additional criteria that can be used to increase the diagnostic accuracy of the US has also been discussed in the literature.[5] For the purpose of this article, the authors will focus on those articles of CTS that elucidate the sensitivity and specificity of the US. The primary objective of the article is to identify sonographic criteria in CTS that can be adhered to in routine clinical practice with ease and accuracy.
Kele et al. described the usefulness of US in evaluating morphological changes in the median nerve and adjacent structures, thus providing a complete imaging picture of CTS.[18] Increased CSA of the median nerve of more than 11 mm2 in the proximal carpal tunnel, at the level of the pisiform, measured in the transverse axis, along with compression of the nerve in the longitudinal axis, gave a sensitivity of 89.1%, specificity of 98%, positive predictive value of 99%, and negative predictive value of 69.8%.
Wong et al. measured the CSA of the median nerve in the transverse axis at three levels in both wrists – proximal to the inlet of carpal tunnel, at the inlet, and the outlet.[2] These levels were identified with reference to the flexor retinaculum. Using 9 mm2 as the cutoff value of CSA of the median nerve proximal to the inlet of the carpal tunnel and 12 mm2 at the outlet, they attained a sensitivity of 94%, specificity of 65%, and false-positive and false-negative rates of 12% and 19%, respectively in the right hand. Using a cutoff value of 10 mm2 proximal to the inlet of carpal tunnel, they reported a sensitivity of 83%, specificity of 73%, and false-positive and false-negative rates of 15% and 31%, respectively, in the left hand. They advocated the use of the US as the preliminary investigation if CTS was being suspected clinically. They also suggested that an electrodiagnostic study should be done only if the US was negative or some additional pathology; besides, CTS was being suspected clinically.
Wiesler et al. measured the CSA of the median nerve at the level of the distal wrist crease.[3] In healthy volunteers, the CSA at this level was 9 mm2, and in patients with CTS, it was 14 mm2. Using a cutoff value of 11 mm2 at this level for the diagnosis of CTS, they attained a sensitivity, specificity, positive predictive value, and negative predictive value of 91%, 84%, 74%, and 95%, respectively. They noted that significant variation in the measurement of median nerve CSA occurred due to the non-standardization of techniques.
Hobson-Webb et al. measured the CSA of the median nerve in the transverse axis at the level of the distal wrist crease and in the forearm, 12 cm proximal to this level, and calculated the wrist-to-forearm ratio (WFR).[19] A WFR value of ≥1.4 yielded a 100% sensitivity in the diagnosis of CTS. The authors postulate that measurement of this ratio would remove variability in measurements due to racial differences and technical factors. It was also reproducible and did not unduly prolong the examination time. They concluded that it would be advantageous to use this ratio in the diagnosis of CTS rather than measuring the nerve CSA just at the level of the wrist.
Klauser et al. measured the CSA of the median nerve at the level of maximum change in its contour as it passed through the carpal tunnel (CSAc) and at the level of proximal one-third of the pronator quadratus muscle in the distal part of the forearm (CSAp).[9] The difference between the two was recorded as Δ CSA. Sensitivity and specificity of 99% and 100%, respectively, were noted in the diagnosis of CTS with Δ CSA of ≥2 mm2. The advantages of using this technique were its high diagnostic accuracy and that it also eliminated the variation in absolute values of CSA found between different individuals depending on their physical and racial characteristics.
Klauser et al. performed another study in which they gave a similar Δ CSA parameter for bifid median nerves.[20] In this study, they added the CSA of the medial and lateral branches of the bifid median nerve in the carpal tunnel (CSAc) and at the level of the upper third of the pronator quadratus muscle in the forearm (CSAp). Subsequently, the difference between these values was calculated, which was given as Δ CSA, and if this was ≥4 mm2, the sensitivity and specificity for diagnosis of CTS were 92.5% and 94.6%, respectively. They concluded that it was the measurement of Δ CSA that increased the sonographic accuracy of the diagnosis of CTS.
Mhoon et al., in their study, correlated measurements of CSA of the median nerve with electrodiagnostic tests.[4] They measured the CSA of the median nerve at the level of the distal wrist crease and about 12 cm proximal to this level and calculated the WFR. Their study revealed a high sensitivity but low specificity for diagnosis of CTS if a value of CSA of >9 mm2 and WFR of 1.4 was used for diagnosis. On analyzing data of those patients who had been diagnosed with CTS on electrodiagnostic tests, they affirmed that sonographic CSA ≥17 mm2 and WFR of ≥2.79 was 100% specific for the same.
McDonagh et al. carried out an analysis of 22 studies that evaluated the role of the US in CTS.[15] Significant variation in the reported sensitivity (62–97.9%) and specificity (57.1–100%) of US in the diagnosis of CTS was noted, and this was attributed to differences in the technique and level of measurement of CSA of the median nerve as well as operator and machine dependence. They suggested that other criteria, such as increased vascularity and hypoechogenicity of the nerve, could be used to increase the accuracy of US. They proposed the use of US as the preliminary imaging modality in patients with clinically suspected CTS and recommended electrophysiological tests in only those patients in whom the US was negative, whose symptoms were atypical, or who did not respond to treatment.
Ng et al. evaluated the utility of measurement of CSA of the median nerve at different levels along with additional parameters such as flattening ratio (FR), vascularity of the nerve, neural fascicular echotexture, and palmar bowing of the flexor retinaculum in the diagnosis of CTS.[5] The different levels at which the nerve CSA was measured were proximal to the inlet of the tunnel (CSAp), at the inlet (CSAi), at the outlet (CSAo), and just distal to the outlet (CSAd). With a CSAp >14 mm2, the sensitivity, specificity, and diagnostic accuracy of US to diagnose CTS were 75%, 87.5%, and 86.8%, respectively. Using a CSAd >14 mm2, the sensitivity, specificity, and diagnostic accuracy of the US were 63.6%, 100%, and 78.9%, respectively. When either CSAp or CSAd >14 mm2 was used to diagnose CTS, the sensitivity and specificity of US were 88.6% and 87.5%. Evaluating CSAd improved the capability of the US in the diagnosis of CTS as the authors found that some patients only had a swollen nerve distal to the outlet but not proximal to the inlet. However, this measurement was difficult due to the thick palmar skin and obliquity and depth of the nerve and needed both a machine that could provide high resolution images as well as technical expertise.
FR of the nerve, which was a ratio of its major axis to minor axis, was calculated at the level of the inlet and outlet of the carpal tunnel. If FR was >2.5 at the inlet, the sensitivity, specificity, and accuracy of US in the diagnosis of CTS were 68%, 45%, and 58.3%, respectively. If palmar bowing of the flexor retinaculum was >1 mm at the outlet, US had a sensitivity, specificity, and accuracy of 72.7%, 96.9%, and 82.9%, respectively. The presence of intraneural vascularity had a sensitivity, specificity, and accuracy of 31.8%, 87.5%, and 55.3%, respectively. Change in echogenicity of the median nerve with loss of the normal fascicular appearance had a sensitivity, specificity, and accuracy of 75%, 90.6%, and 81.6%, respectively. After evaluation of all these parameters, the authors concluded that when the diagnosis of CTS was based on either CSAp or CSAd >14 mm2 or palmar bowing of the flexor retinaculum at the outlet >1 mm, US had high sensitivity, specificity, and accuracy which were 100%, 84.3%, and 93.4%, respectively. Other criteria, such as FR, presence of intraneural vascularity, and loss of the neural fascicular appearance, were not found to be significantly useful in diagnosing CTS.
Ratasvuori et al. correlated measurements of CSA of the median nerve with clinical findings and electrodiagnostic tests.[21] They measured the CSA of the median nerve at two levels – 10–12 cm proximal to the wrist crease and just proximal to the carpal tunnel inlet. With a CSA cutoff value of 11.5 mm2 proximal to the carpal tunnel inlet, they attained a sensitivity of 87% and specificity of 91% in the US diagnosis of CTS. They found that the calculation of the WFR did not result in an increased sensitivity or specificity of the US.
A summary of the sensitivity and specificity of different studies evaluating the role of the US in the diagnosis of CTS is given in Table 1. The variability in criteria used for diagnosis is clear from one glance at the table.
| Study | Criteria | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Kele et al. | Increased nerve CSA in the proximal carpal tunnel >11 mm2 | 89.1 | 98 |
| Wong et al. | >9 mm2 CSA proximal to an inlet of carpal tunnel | 94 | 65 |
| Wiesler et al. | >11 mm2 at the level of the distal wrist crease | 91 | 84 |
| Hobson-Webb et al. | Nerve CSA at the level of distal wrist crease and 12 cm proximal to this level, WFR ≥1.4 | 100 | |
| Klauser et al. | Nerve CSA in the carpal tunnel and at the level of proximal third of pronator quadratus, difference recorded as ΔCSA, ΔCSA ≥2 mm2 | 99 | 100 |
| Mhoon et al. | Nerve CSA at the level of distal wrist crease and 12 cm proximal to this level, CSA >17 mm2 and WFR ≥2.79 | 100 | |
| Ng et al. | CSA proximal to the inlet or distal to the outlet >14 mm2 | 88.6 | 87.5 |
| FR >2.5 at the inlet | 68 | 45 | |
| Palmar bowing of flexor retinaculum >1 mm at outlet | 72.7 | 96.9 | |
| Intraneural vascularity | 31.8 | 87.5 | |
| Change in nerve echogenicity | 75 | 90.6 | |
| Either CSA proximal to inlet or distal to outlet >14mm2 or palmar bowing of flexor retinaculum >1 mm at the outlet | 100 | 84.3% | |
| Ratasvuori et al. | CSA proximal to the inlet >11.5mm2 | 87 | 91 |
CSA: Cross-section area, WFR: Wrist-to-forearm ratio, FR: Flattening ratio
RECOMMENDATIONS
After an in-depth analysis of the various US criteria used to measure the CSA of the median nerve mentioned in these studies on CTS, the authors believe that in daily clinical practice, measurement of Δ CSA (Klauser et al.) gave the best results.[9] In this study, the authors measured CSAc, which is the CSA of the median nerve at the level of maximal contour change as the nerve traversed the carpal tunnel, and CSAp, which is the CSA at the level of proximal third of pronator quadratus muscle. When the difference in these values, Δ CSA, is ≥2 mm2, the patient is diagnosed with CTS based on the US findings. However, if there are symptoms of nerve entrapment but the nerve is not enlarged proximally, then the next step is to measure the CSAd, which is the CSA of the median nerve distal to the outlet (Ng et al.).[5] If the CSAd is >14 mm2, it is considered diagnostic of CTS.
In the author’s opinion, it would be prudent to use Klauser’s method and also measure the CSAd by Ng et al., which would identify patients who have compression both at the inlet and outlet of the carpal tunnel.[5]
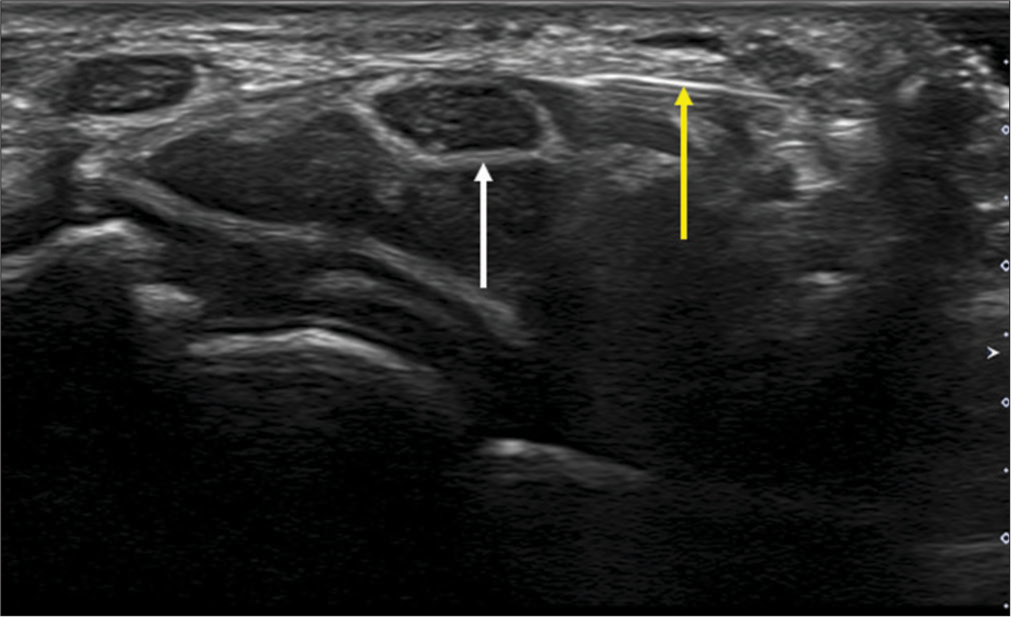
In addition, the US study should mention any change in the echogenicity of the nerve, including its fascicular appearance, presence of intraneural vascularity, and bowing of the flexor retinaculum [Figures 4 and 5]. Any anatomical variations such as a bifid median nerve, persistent median artery, superficial ulnar artery, or anomalous flexor digitorum superficialis muscle must be reported [Figure 6]. The presence of variation in the division of the median nerve proximal to the outlet, including the recurrent motor branch of the median nerve, needs to be detailed to provide the surgeon with this information preoperatively.[22]
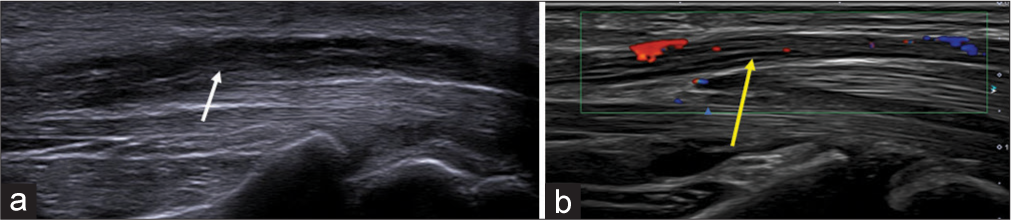
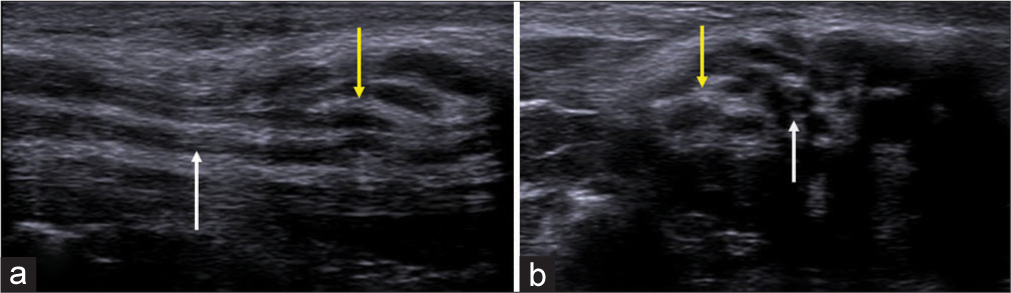
- (a) Longitudinal axis ultrasound image of the median nerve showing thickened hypoechoic nerve fascicles (white arrow) and (b) Doppler image showing intra-neural vascularity (yellow arrow).
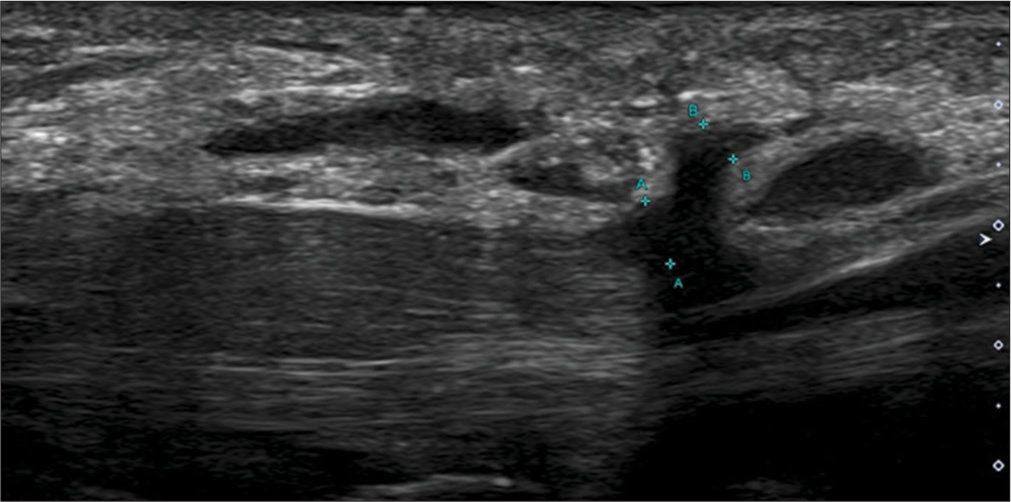
- Longitudinal axis ultrasound image showing the thickened and hypoechoic recurrent motor branch of median nerve (indicated by green calipers) in a patient with neuropathy. The median nerve is also grossly thickened.
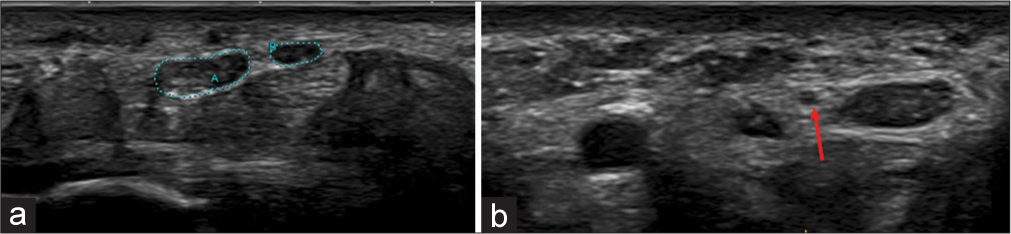
- (a) Transverse axis ultrasound image showing bifid median nerve, outlined by green dots and in a different patient, (b) the persistent median artery (red arrow) is seen between the bifid median nerves.
Neurogenic or non-neurogenic mass lesions in the carpal tunnel, such as fibrolipomatous hamartoma or lipoma and inflammatory or infective tenosynovitis, which can cause nerve compression, need to be enumerated [Figures 7 and 8]. Nerve involvement with subsequent secondary compression in the tunnel can occur due to infective causes such as leprosy, herpes, or COVID-19 [Figure 9].[23-26] Identification of the etiology of CTS by the US will help to establish the diagnosis and also facilitate surgical planning by the treating plastic surgeon.
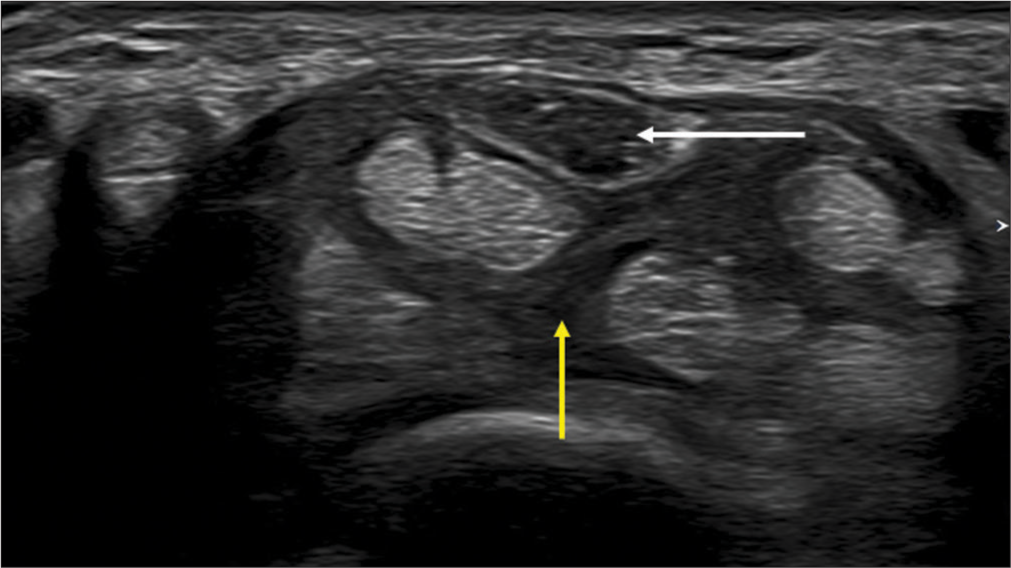
- (a) Longitudinal axis and (b) transverse axis ultrasound images in a patient with fibrolipomatous hamartoma of the median nerve showing the enlarged nerve with thickened hypoechoic fascicles (white arrows) surrounded by echogenic fat (yellow arrows).
- Transverse axis ultrasound image showing median neuropathy (white arrow) due to flexor tenosynovitis (yellow arrow).
- Longitudinal axis ultrasound image in a patient with leprosy showing an enlarged median nerve with thickened hypoechoic fascicles (white arrow).
Recent studies on CTS have evaluated the mobility and stiffness of the median nerve. However, a detailed study of these techniques is beyond the scope of this paper.
At present, the US also plays a major role in treating CTS patients with its ability to guide procedures such as perineural corticosteroid injection, hydro dissection, and minimally invasive carpal tunnel release [Figure 10].[27-33] Thus, it helps to ameliorate the patient’s symptoms cost-effectively.
- Transverse axis ultrasound image showing hydrodissection needle (yellow arrow) in a case of median neuropathy (white arrow).
CONCLUSION
When diagnosing CTS, US offers a high degree of sensitivity, specificity, and accuracy. The criteria used to diagnose CTS in daily practice should be simple and accurate without significant inter-observer variability. For the diagnosis of CTS, the authors recommend using the Klauser’s method of a Δ CSA of ≥2mm2 when the nerve CSA was measured in the carpal tunnel and at the level of the proximal third of the pronator quadratus muscle. If this is combined with Ng’s method of CSA distal to the outlet of more than 14 mm2, then cases of compression of the median nerve at the inlet and outlet can be identified with greater sensitivity, specificity, and accuracy. Using EMG correlation would be helpful when US findings are borderline positive for CTS. Reporting other US characteristics is encouraged but not mandatory in the conclusion of the interpretation. However, a detailed description of the normal variations of the median nerve and persistent median artery is essential for purposes of surgery.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Carpal tunnel syndrome In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.nabiullah.nam.nih.gov/books/nbk448179 [Last accessed on 2024 Jun 18]
- [Google Scholar]
- Carpal tunnel syndrome: Diagnostic usefulness of sonography. Radiology. 2004;232:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726-32.
- [CrossRef] [PubMed] [Google Scholar]
- Median nerve ultrasound as a screening tool in carpal tunnel syndrome: Correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle Nerve. 2012;46:871-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound carpal tunnel syndrome: Additional criteria for diagnosis. Clin Radiol. 2018;73:214.e11-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography in the diagnosis of carpal tunnel syndrome: A critical review of the literature. Muscle Nerve. 2003;27:26-33.
- [CrossRef] [PubMed] [Google Scholar]
- MRI criteria for diagnosis and predicting severity of carpal tunnel syndrome. Skeletal Radiol. 2020;49:397-405.
- [CrossRef] [PubMed] [Google Scholar]
- MRI of the median nerve in carpal tunnel syndrome. J Peripher Nerve Surg. 2020;4:15-21.
- [Google Scholar]
- Carpal tunnel syndrome assessment with the US: Value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound assessment on selected peripheral nerve pathologies. Part I: Entrapment neuropathies of the upper limb-excluding carpal tunnel syndrome. J Ultrason. 2012;12:307-18.
- [CrossRef] [PubMed] [Google Scholar]
- The peripheral nerves: Update on ultrasound and magnetic resonance imaging. Clin Exp Rheumatol. 2018;36:145-58.
- [Google Scholar]
- Nerve entrapment syndromes of the elbow, forearm, and wrist. AJR Am J Roentgenol. 2010;195:585-94.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med. 2016;35:683-93.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic potential of ultrasound in carpal tunnel syndrome with different etiologies: Correlation of sonographic median nerve measures with electrodiagnostic severity. BMC Musculoskelet Disord. 2019;20:634.
- [CrossRef] [PubMed] [Google Scholar]
- The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology (Oxford). 2015;54:9-19.
- [CrossRef] [PubMed] [Google Scholar]
- Echotexture of peripheral nerves: Correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291-6.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging in peripheral neuropathy: Ultrasound and MRI. Indian J Musculoskeletal Radiol. 2021;3:14-23.
- [CrossRef] [Google Scholar]
- The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology. 2003;61:389-91.
- [CrossRef] [PubMed] [Google Scholar]
- The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bifid median nerve in carpal tunnel syndrome: Assessment with US cross-sectional area measurement. Radiology. 2011;259:808-15.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography for the diagnosis of carpal tunnel syndrome: Correlation of clinical symptoms, cross-sectional areas and electroneuromyography. J Hand Surg Eur Vol. 2022;47:369-74.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variations of the carpal tunnel structures. Can J Plast Surg. 2009;17:e3-7.
- [CrossRef] [Google Scholar]
- Tingling hand: Magnetic resonance imaging of median nerve pathologies within the carpal tunnel. Pol J Radiol. 2019;84:e484-90.
- [CrossRef] [PubMed] [Google Scholar]
- The distribution of acquired peripheral nerve injuries associated with severe COVID-19 implicate a mechanism of entrapment neuropathy: A multicenter case series and clinical feasibility study of a wearable, wireless pressure sensor. J Neuroeng Rehabil. 2022;19:108.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes zoster in the median nerve distribution. J Plast Reconstr Aesthet Surg. 2010;63:e195-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of herpes zoster infection mimicking carpal tunnel syndrome. Neurol India. 2019;67:272-3.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided peripheral nerve injection techniques. AJR Am J Roentgenol. 2016;207:507-16.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided corticosteroid injection in carpal tunnel syndrome: Comparison between radial and ulnar approaches. J Pain Res. 2020;13:1569-78.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness and safety of commonly used injectates for ultrasound-guided hydrodissection treatment of peripheral nerve entrapment syndromes: A systematic review. Front Pharmacol. 2021;11:621150.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided hydrodissection with corticosteroid injection in the treatment of carpal tunnel syndrome: A pilot study. J Ultrasound Med. 2020;39:1759-68.
- [CrossRef] [PubMed] [Google Scholar]
- Dexamethasone versus hyaluronidase as an adjuvant to local anesthetics in the ultrasound-guided hydro dissection of the median nerve for the treatment of carpal tunnel syndrome patients. Anesth Essays Res. 2019;13:417-22.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided nerve hydro dissection for pain management: Rationale, methods, current literature, and theoretical mechanisms. J Pain Res. 2020;13:1957-68.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive ultrasound-guided carpal tunnel release improves long-term clinical outcomes in carpal tunnel syndrome. AJR Am J Roentgenol. 2021;217:460-8.
- [CrossRef] [PubMed] [Google Scholar]






