Translate this page into:
Ribbing disease- radiograph to the rescue in an overlooked cause of painful shin

*Corresponding author: Suvinay Saxena, Department of Diagnostic and Interventional Radiology, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India. suvinaysaxena@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saxena S, Yadav T, Jha S, Khera PS. Ribbing disease- radiograph to the rescue in an overlooked cause of painful shin. Indian J Musculoskelet Radiol 2021;3:111-6.
Abstract
Ribbing disease or multiple diaphyseal sclerosis is a rare clinical entity, which is often confused with many other differential diagnoses when it primarily involves the tibia and fibula. An accurate diagnosis alleviates the need for tissue diagnosis and aids in better clinical management and outcome of the patient. The case report highlights the classical imaging findings, appearance on cross-sectional imaging, and the admirable role of radiographs in diagnosing the entity.
Keywords
Ribbing disease
Multiple diaphyseal sclerosis
Radiograph
Magnetic resonance imaging
INTRODUCTION
Hereditary multiple diaphyseal sclerosis (MDS) or Ribbing disease is an uncommon form of sclerosing bone dysplasia that has a strong predilection for involvement of the long tubular bone. It was named after Dr. S. Ribbing, who first described this condition in 1949. This condition is found typically in postpubertal females, though cases have been reported in male patients and usually present in and after the third decade of life. We present an interesting case of a young gentleman referred to the diagnostic imaging department with the clinical suspicion of osteomyelitis and was finally diagnosed with Ribbing disease.[1]
CASE REPORT
A 32-year-old man, a police constable by occupation presented to the department of diagnostic and interventional radiology for review evaluation of cross-sectional imaging (done outside)of the right leg with a clinical suspicion of osteomyelitis. The patient had a history of deep-seated pain in the right leg for 5 months (March 2020). No history of direct trauma or fever was present. Clinical examination of the patient was unremarkable except for slight tenderness along the right leg over tibial prominence. The patient had a normal range of motion of the knee and hip joint. Hemogram and inflammatory markers were essentially normal. (Total leucocyte count-7710 c/ul, Erythrocyte sedimentation rate [ESR] 24 mm/h, C-reactive protein 4 mg/ml). Serum calcium 9.5 mg/dl, Serum Phosphorus 3.30 mg/dl, and Alkaline Phosphatase (ALP) was 90 U/dl.
Magnetic resonance imaging (MRI) films revealed circumferential periosteal thickening with near-complete obliteration of the medullary cavity along the mid-shaft of the right tibia, which was hypointense on T2 weighted images, short tau inversion recovery (STIR) coronal images reveal subtle edema along with the adjacent marrow. The above findings were corroborated on Computed Tomography (CT) [Figure 1]. However, extensive sclerosis was also seen along the entire mid and distal shaft of the fibula. Due to ipsilateral multiple bone involvement, lack of adjacent inflammation along with the soft tissues and no history of trauma, differentials of a stress fracture, sclerosing osteomyelitis, or proliferative bone tumor-like osteosarcoma were provisionally excluded from the study.
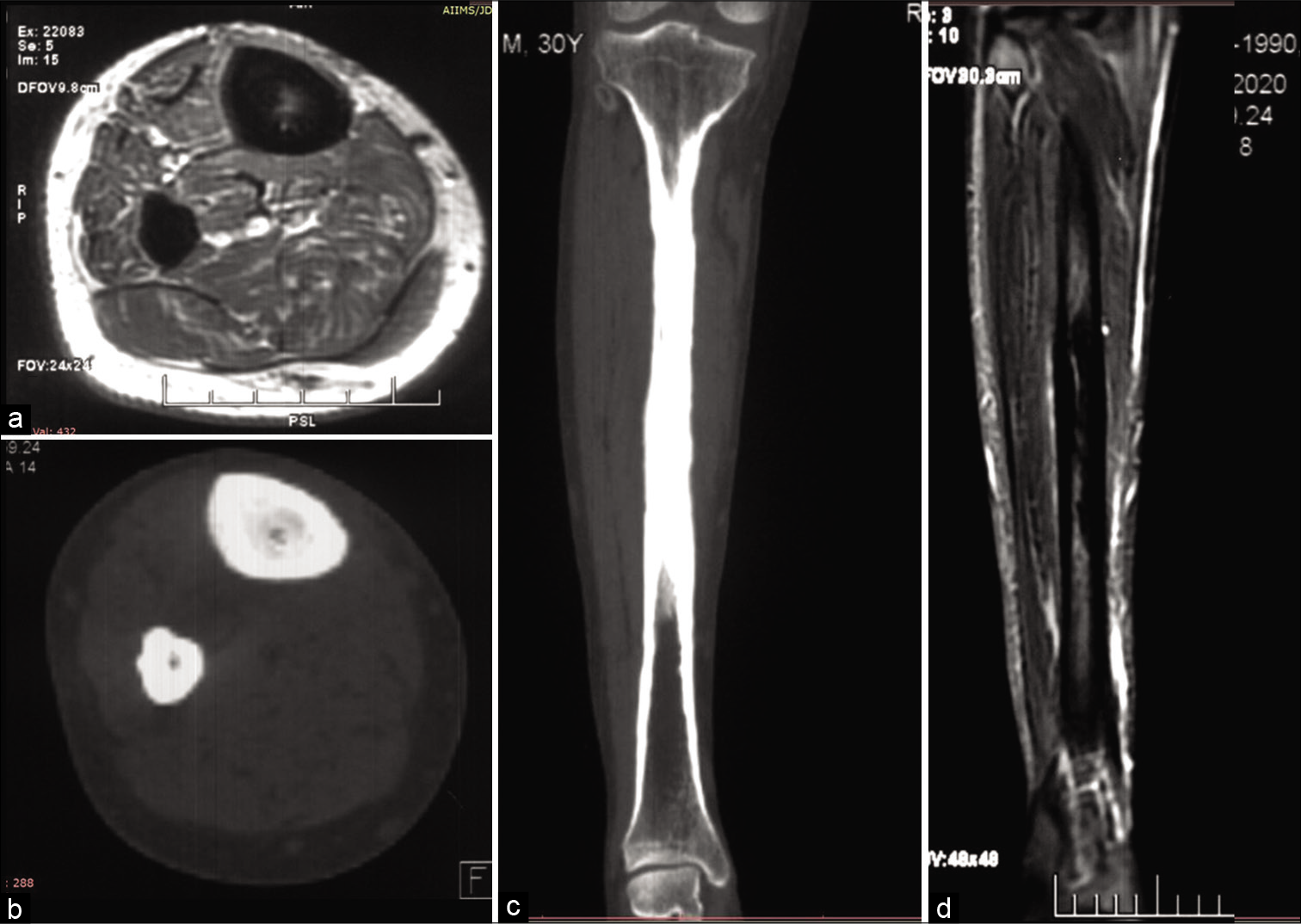
- Magnetic resonance imaging (MRI) T1 weighted axial images of the right leg depict hypointense circumferential periosteal thickening with medullary narrowing (a) Corresponding axial computed tomography (CT) (b) and coronal CT (c) bone window confirmed the findings of the leg, MRI coronal short tau inversion recovery images (d) depict edema as hyperintensity along the midshaft of the tibia.
A skeletal survey was done due to high suspicion of sclerosing dysplasia. The frontal radiograph of the right leg revealed excessive cortical periosteal thickening predominantly along the tibial and fibular diaphysis with sparing of the metaphysis. No evident cortical break or fracture was seen. Similar exuberant changes were observed along the mid-shaft of the left fibula and mild periosteal reaction along the left tibia [Figure 2]. Skeletal survey further revealed exuberant periosteal and endosteal new bone formation along mid-shaft of the bilateral femur, bilateral radial and ulnar mid shafts with sparing of distal ends [Figure 3]. Evaluating the lateral skull radiograph and frontal radiograph of the hand and wrist revealed mild periosteal thickening along bilateral metaphysis of second and third metacarpals and proximal phalanges [Figure 4], Frontal and lateral spine radiographs, bilateral humeri, and ribs were unremarkable [Figure 5].
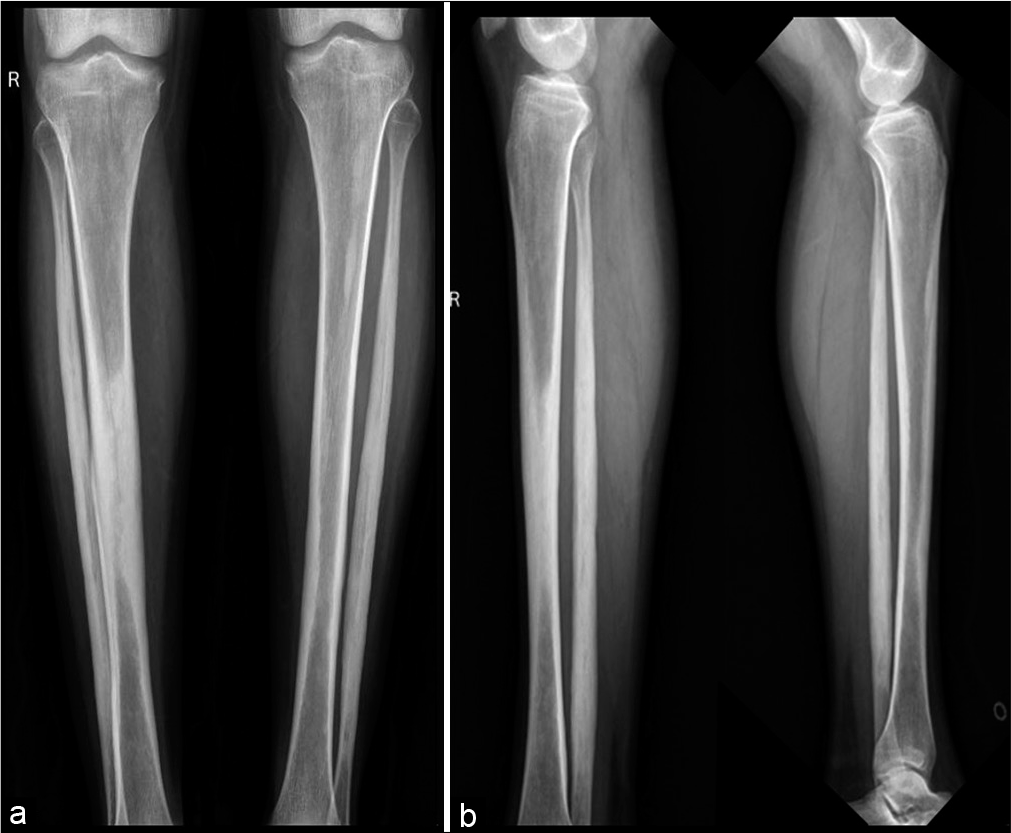
- Frontal and lateral radiograph of both legs showing extensive periosteal reaction along the midshaft of right tibia, bilateral fibular shafts with sparing of distal ends. Note was made of early periosteal reaction along the left tibial midshaft.
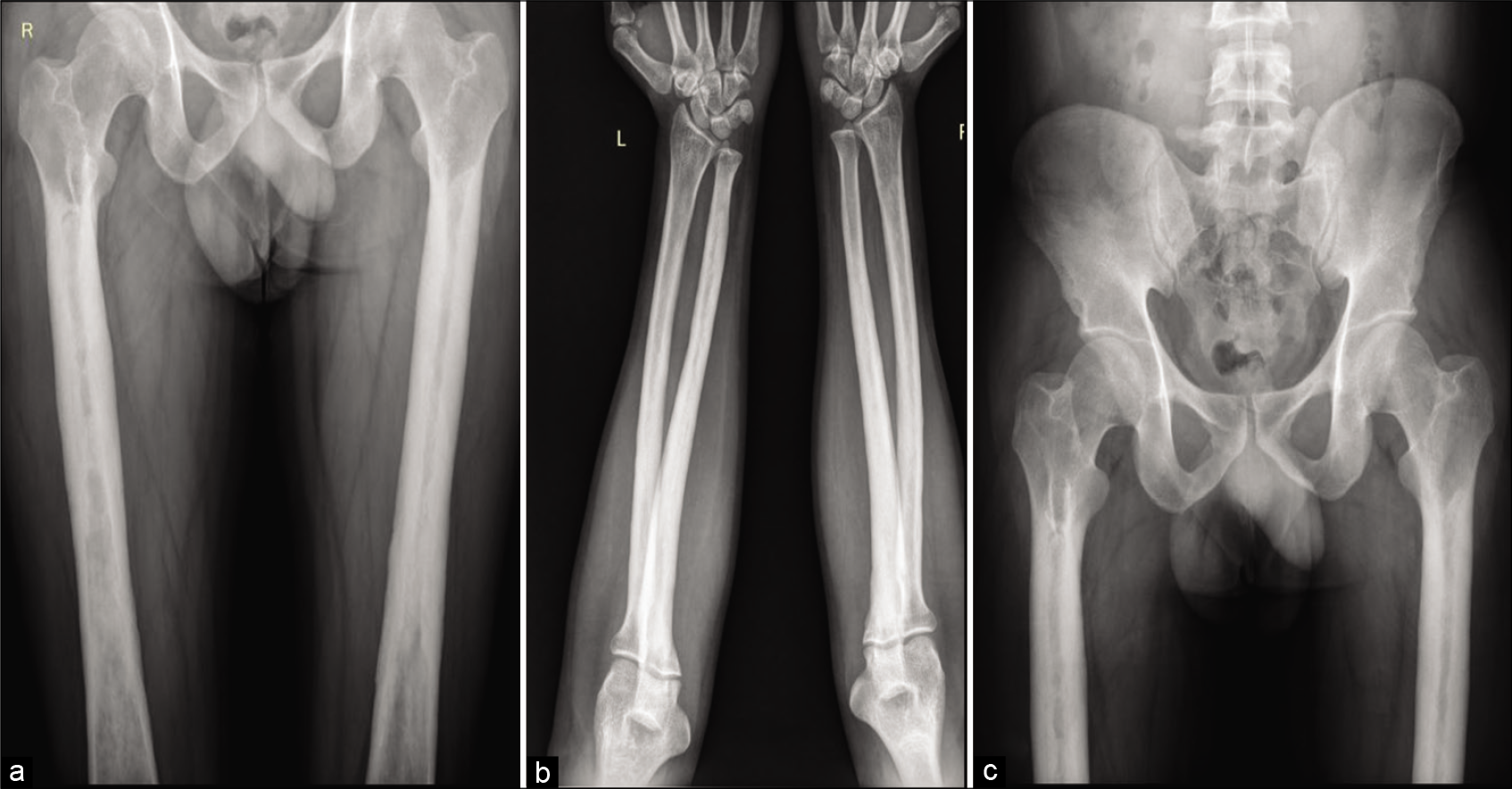
- Frontal radiograph of both femori (a), bilateral elbow with forearm anterior-posterior (AP) radiograph (b), and pelvis AP radiograph (c) shows circumferential periosteal thickening with medullary narrowing along the proximal and midshaft of bilateral femori and proximal and midshaft radius and ulna.
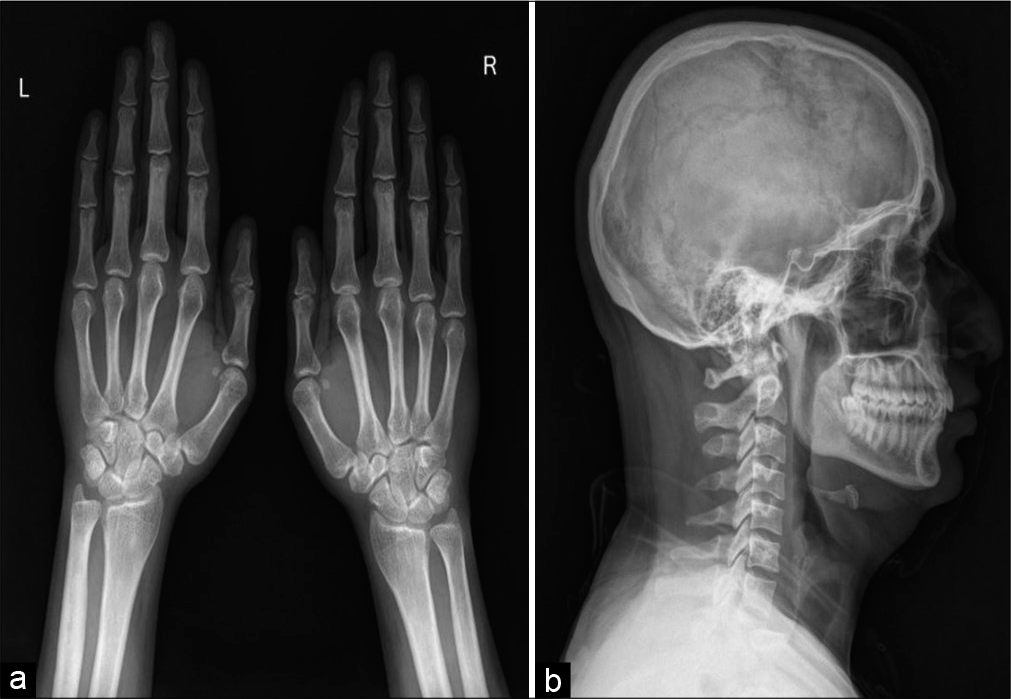
- Frontal radiograph of both wrist with hands (a) and skull lateral radiograph (b) shows circumferential periosteal thickening with medullary narrowing along bilateral second metacarpal and bilateral 3rd proximal phalanges.
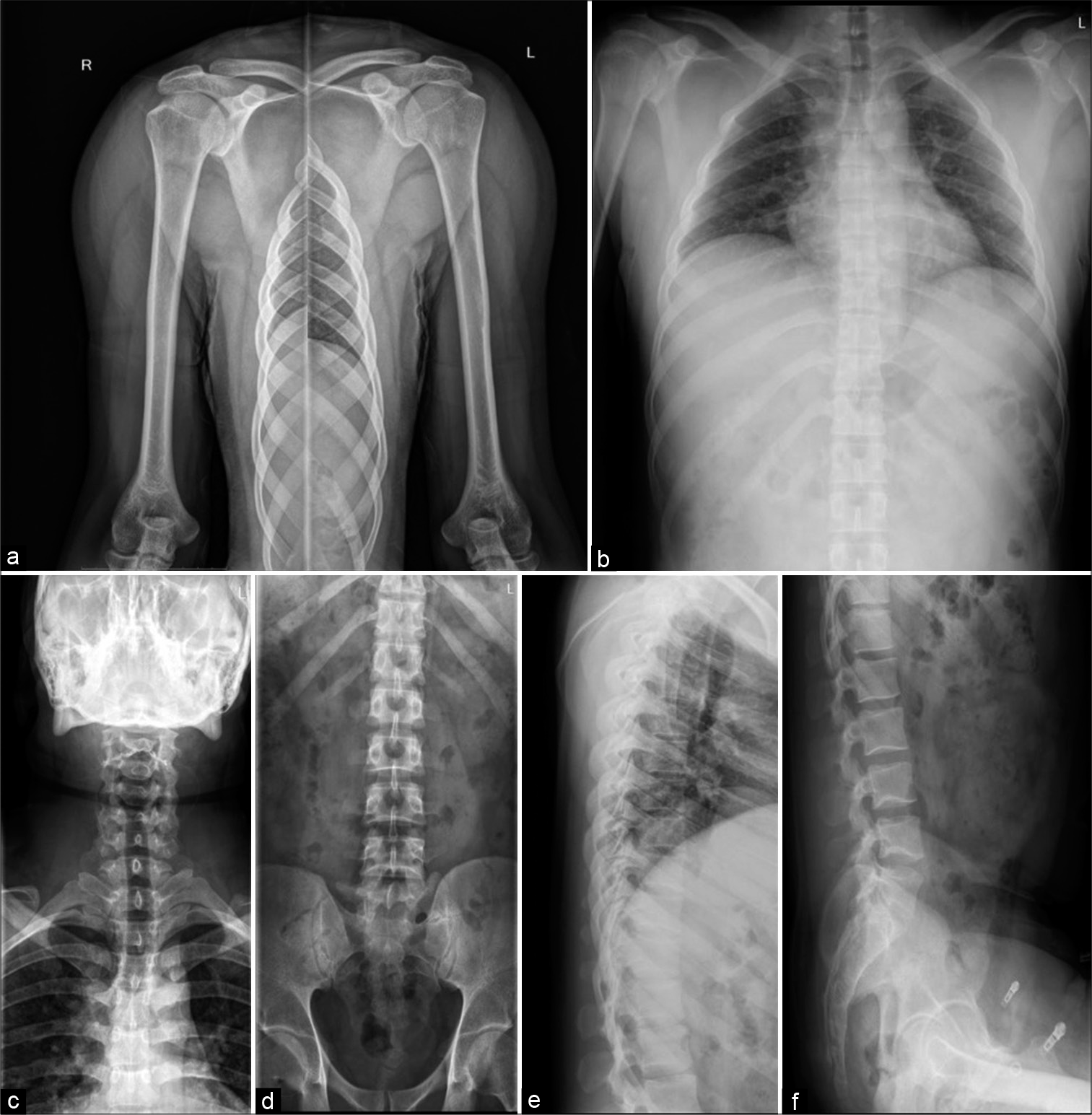
- Frontal radiograph of both shoulder with humeri (a), Dorsal spine anterior-posterior (AP) (b) and Lateral view (e), Cervical spine AP (c), Lumbar spine AP (d), and lateral (f) radiographs appear unremarkable.
DISCUSSION
Patients with hereditary MDS usually present with longstanding diaphyseal pain of lower limbs with a duration of pain ranging between 1 and 16 years.[2,3] The radiographs often show unilateral or bilateral, asynchronous increased sclerosis along the diaphysis with an endosteal, periosteal, and cortical thickening. This progresses with time and spreads longitudinally and can be associated with the narrowing and obliteration of the medullary canal. Epiphyses and metaphysis, however, are typically spared. The bone scintigraphy (Technetium-99-methylene diphosphonate) shows an increased uptake, at sites corresponding to areas of sclerosis on plain radiographs and clinically correlates with areas of pain. Laboratory findings such as ESR, complete blood count, and ALP are within normal ranges. CT scan can substantiate the radiographic findings with a better depiction of intramedullary narrowing. The MRI examination confirms the presence of sclerosis and when the pain is presenting a feature, bone marrow edema can be documented on (STIR) sequences and is usually present along the mid-shaft of the affected bones.[4]
A close differential diagnosis of this condition is progressive diaphyseal dysplasia, also known as Camurati-Engelmann disease, which is primarily seen in childhood and prepubertal age groups.[2,4] The primary features which aid in differentiation include the absence of skull base involvement in Ribbing disease, unlike Camurati-Engelmann disease. Other differences include more symmetrical bone involvement, progression to the metaphysis, and presence of mandible involvement, continuous progression, gait abnormalities leading to disability, neuromuscular symptoms in some of the cases of Camurati- Engelmann disease.[2,4,5]
Another close differential is hyperostosis corticalis generalisata, which is a dysplasia resulting from abnormal intra-membranous ossification[2,5] and is seen in childhood. The presence of predominant skull base and mandible involvement, with or without the involvement of long bones is an important differentiating feature. Pyknodysostosis forms an important differential which contrary to Ribbing disease, is seen in early infancy and childhood with preservation of the medullary cavity. It is also associated with dwarfism, acroosteolysis, and presence of Wormian bones, calvarial thickening, nasal beaking, and obtuse mandibular gonial angle.[6,7] Erdheim-Chester Disease or non-Langerhans cell histiocytosis is a close differential with diaphyseal involvement, narrowing of the medullary cavity, and sparing of epiphyses. However, unlike Ribbing disease, the latter involves metaphysis along with diaphysis with bilateral symmetrical involvement, bone infarcts may be seen in this condition, along with multiple clinical extraosseous manifestations such as pulmonary fibrosis, heart failure, diabetes insipidus, painless bilateral exophthalmos, hydronephrosis, and chronic renal failure.[7,8] Cortical and medullary hyperostosis of a single bone or multiple adjacent bones with a flowing ‘dripping candle wax’ appearance is classical of melorheostosis. In osteopetrosis, there is the involvement of all components of long bone including metaphysis and diaphysis in addition to axial skeleton involvement [Table 1].[9]
| Clinical entity | Demographics | Pattern of inheritance | Imaging features | |
|---|---|---|---|---|
| Predominant involvement | Findings | |||
| Hereditary multiple diaphyseal sclerosis/ Ribbing disease | Post puberty | Autosomal Recessive (AR) | Exclusively long bones Diaphysis involved Spring of epiphyses and metaphysis | Uni/bilateral Asynchronous Progressive sclerosis along diaphysis with endosteal, periosteal, and cortical thickening, increases longitudinally May be associated with narrowing and obliteration of the medullary canal |
| Progressive diaphyseal dysplasia/Camurati Engelman disease | Pre-puberty (childhood) | Autosomal dominant (AD) | Long bones (diaphysis>metaphyses) Skull bones | Bilateral Symmetric Periosteal and endosteal cortical thickening Progressive to involve metaphyses in later stages |
| Hyperostosis cortica genralisata | Childhood | AR (van buchem disease sclerosteosis) or AD (worth disease) | Skull base and mandible predominant involvement Long bones ± | Endosteal cortical thickening involving the skull, and facial bones; mandible enlargement, and/or long bones |
| Pyknodysostosis | Early infancy and childhood | AR | Skull, facial bones, clavicles, long bones, fingers, vertebrae | Hyperostosis of long bones with preserved medullary canal |
| Erdheim-Chester disease or non-Langerhans cell histiocytosis | adulthood | – | Long bones diaphysis and meteaphyses sparing of epiphyses | Bilateral Symmetrical Diaphyseal sclerosis with narrowing of the medullary cavity |
| Melorheostosis | Early childhood to early adulthood | – | Long bones | Cortical and endosteal thickening with medullary sclerosis thick undulating ridges of bone |
| Osteopetrosis | Infancy/adulthood | AR – Infant AD - Adult |
Long bone Epi-metaphysis and diaphysis Axial skeleton |
Diffusely increased bone density; uniform sclerosis of the skull, spine, and long bones; or bone with in bone appearance |
Our patient has been initiated on analgesics, bisphosphonates, surgical treatment options have been explained to the patient, and is on medical follow-up. There is a paucity of literature about treatment options which include a gamut of pharmaceutical agents including non-steroidal anti-inflammatory drugs and prednisone. Many studies have documented the use of bisphosphonates but there is overlap in outcome and efficacy. Surgical interventions in the form of either reaming or fenestrations are aimed at relieving the intramedullary pressure, which is believed to be the etiopathology behind the pain.[9,10]
CONCLUSION
A structured clinical-radiological approach to all patients presenting with sclerosis is crucial for a specific diagnosis for such a “Do not touch” bony lesion. Early diagnosis of Ribbing disease aids in early intervention and treatment thus alleviating the long painful years a patient may have to endure due to an improper diagnosis. A holistic multi-modality approach is essential for diagnosis, but radiographs form the backbone of the algorithm.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hereditary, multiple, diaphyseal sclerosis. Acta Radiol. 1949;31:5-6, 522-36
- [CrossRef] [PubMed] [Google Scholar]
- Ribbing disease: A systematic review. Acta Radiol. 2018;59:448-53.
- [CrossRef] [PubMed] [Google Scholar]
- Ribbing disease (multiple diaphyseal sclerosis): Imaging and differential diagnosis. AJR Am J Roentgenol. 1996;167:689-94.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple diaphyseal sclerosis (Ribbing disease): What about neridronate? Osteoporos Int. 2016;27:3127-31.
- [CrossRef] [PubMed] [Google Scholar]
- Sclerosing bone dysplasias-a target-site approach. Skeletal Radiol. 1991;20:561-83.
- [CrossRef] [PubMed] [Google Scholar]
- Sclerosing bone dysplasias: Review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31:1865-82.
- [CrossRef] [PubMed] [Google Scholar]
- Ribbing disease: Uncommon cause of a common symptom. Indian J Nucl Med. 2011;26:36-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ribbing disease: Radiographic and biochemical characterization, lack of response to pamidronate. Skeletal Radiol. 2002;31:714-9.
- [CrossRef] [PubMed] [Google Scholar]






