Translate this page into:
Toxic flare phenomenon in osteoblastoma: A case report with literature review

*Corresponding author: Vikas Batra, Department of Radiology, Mahajan Imaging SIC, Safdarjung Hospital, New Delhi, India. drvikas28@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Batra V, Batta NS, Gupta A. Toxic flare phenomenon in osteoblastoma: A case report with literature review. Indian J Musculoskelet Radiol 2021;3:106-10.
Abstract
Osteoblastomas are benign neoplasm of the bone that is characterized by clinical and histological similarity to osteoid osteomas. They are larger (>1.5–2 cm) and tend to affect the axial skeleton. Patients typically present commonly in the second or third decades of life with a male predilection. The flare phenomenon in osteoblastoma is a rare but known entity showing florid multifocal periostitis. Few cases have been reported in the literature. Focally aggressive osteoblastomas also present with flare phenomenon mediated by prostaglandin-induced inflammation. We report a strikingly unusual case of an osteoblastoma of the spine in a 23-year-old male patient who presented in our outpatient department with complaints of low backache, weight loss, intermittent stiffness, and fever for the past year. The patient was HLA-B27 positive, diagnosed as ankylosing spondylitis, and clinical symptoms were attributed to sacroiliitis. However, his fever and weight loss, while on treatment, were unexplained. Roentgenogram, computed tomography, and magnetic resonance imaging of the patient depicted a large bone tumor centered at the junction of the right pedicle and transverse process of L5 vertebra, associated with severe reactive marrow edema, periosteal reaction, periostitis, and sclerosis in the posterior vertebral elements also extending into the L4 vertebra. The lesion was suspected to be osteoblastoma with close imaging differential as osteosarcoma. CT-guided biopsy proved osteoblastoma with acute local toxic flare.
Keywords
Vertebral osteoblastoma
Toxic flare
Bone tumor
Ankylosing spondylitis
INTRODUCTION
Osteoblastomas are benign bone-forming neoplasm comprising 1–3% of all primary bone tumors, characterized by trabeculae of osteoid, and woven bone with interspersed vascular spindle fibroblastic stroma (clinical and histological similarity to osteoid osteomas).[1] They are larger >1.5– 2 cm, hence called giant osteoid osteoma, and tend to affect the axial skeleton. There is definite male predilection (2:1), and patients typically present in the second to third decades of life.[1,2] Systemic toxic flare phenomenon in the osteoblastoma is a rarely reported entity in the literature which is radiologically seen as florid multifocal periostitis. Some of the similar cases were seen in preteen children 5–12 years,[3] and another case was seen in an 18-year-old male patient.[4]
We present an unusual case of locally aggressive spinal osteoblastoma in a young adult showing toxic local flare phenomenon with acute inflammatory sacroiliitis in human leukocyte antigen (HLA)-B27 positive, known case of ankylosing spondylitis (AS).
CASE REPORT
A 23-year-old male patient with low backache, intermittent fever, malaise, weight loss, and stiffness for the past 1 year visited our orthopedic outpatient department. Clinical examination revealed tenderness over the lower back at the L5 vertebral level, focal scoliosis with decreased range of movements, and pain during flexion. The patient was advised serological and radiological investigations given high clinical suspicion for ankylosing spondylitis - AS, and test results were positive for HLA-B27.
A plain radiograph of the lumbosacral spine revealed an expansile sclerotic lesion in the right posterior neural elements of the L5 vertebra associated with right facetal arthropathy and focal scoliotic deformity, as depicted in [Figure 1]. Computed tomography (CT) and magnetic resonance imaging (MRI) were advised for better and further delineation of the lesion.
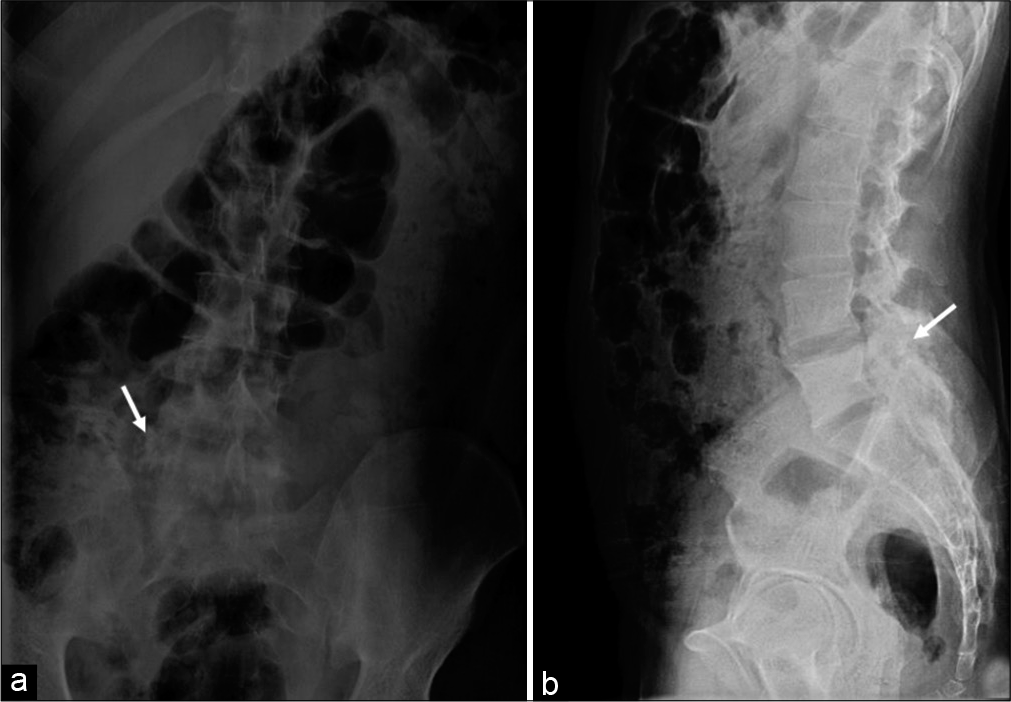
- A 23-year-old male who presented with low backache, stiffness, and intermittent fever for past 1 year. Findings: Anteroposterior (a) and lateral (b) radiographs reveal heterogeneous expansile sclerotic lesion (arrow) in the right pedicle of L5 vertebra also involving the transverse process associated with the right facetal arthropathy.
CT (16 slices GE Medical Systems) was performed with spiral acquisition and images were reformatted in axial, coronal, and sagittal planes.
CT revealed a large heterogeneous expansile well-demarcated predominantly blastic lesion with internal speckled mineralization centered at the junction of the right pedicle and transverse process of L5 vertebra. Significant perilesional sclerosis was noted extending to the ipsilateral lamina, left pedicle, lamina, and vertebral body. The lesion showed partial encroachment into the spinal canal and compression of the thecal sac. Focal areas of cortical breach, right paravertebral soft-tissue ossification, periostitis, and sclerosis were noted in the body, bilateral pedicles, and spinous process of L5 vertebra. There was associated right facet hypertrophic arthropathy at L4-L5 and L5-S1 levels and sclerosis extending into the posterior aspect of the L4 vertebral body [Figure 2a-c].
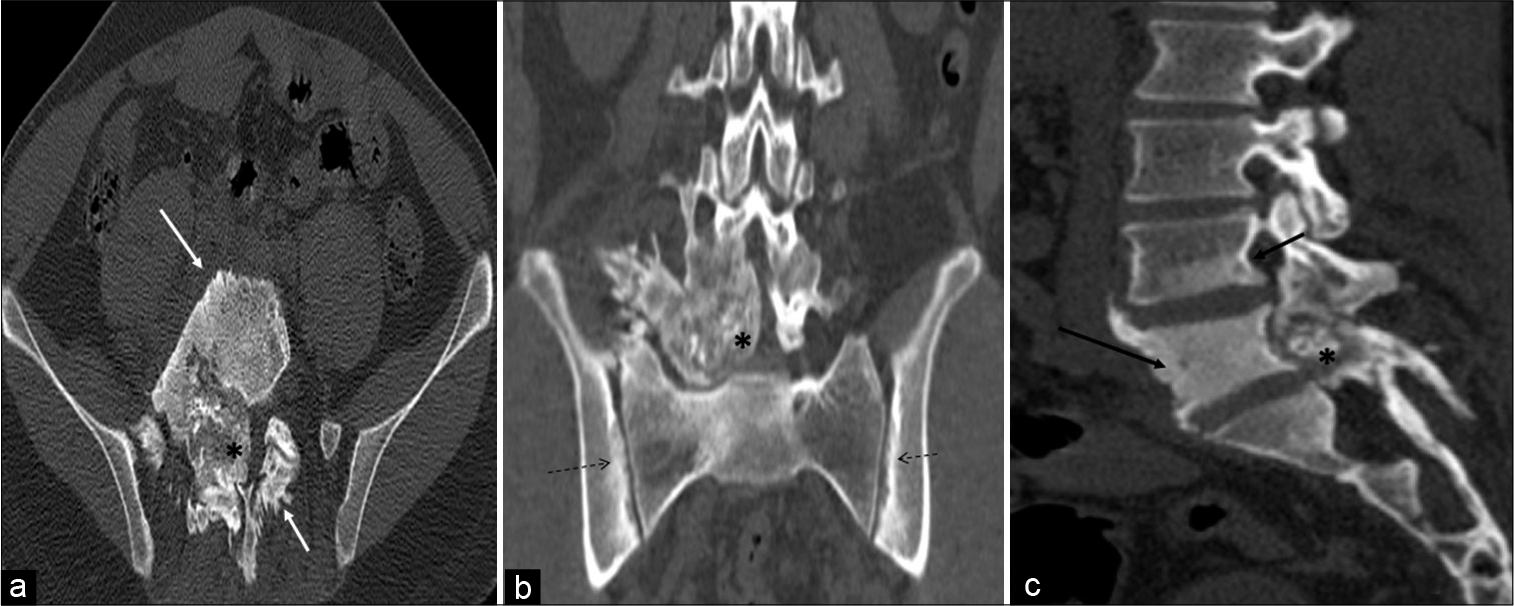
- A 23-year-old male who was diagnosed with osteoblastoma showing toxic flare and coexistent HLA-B27-related ankylosing spondylitis. Axial (a), coronal (b), and sagittal (c) computed tomography multiplanar reformations showing heterogeneous expansile predominantly blastic/sclerotic lesion (asterisk) with internal speckled mineralization centered at the junction of right pedicle and transverse process of L5 vertebra. There is extensive periostitis/periosteal reaction (white arrow) mimicking an aggressive neoplasm. Sclerosis is seen in the entire L5 and inferior aspect of the L4 vertebra (black arrow). There is associated bilateral symmetrical sacroiliitis (dashed arrows).
Changes of bilateral symmetrical sacroiliitis were also seen in the form of irregularity and erosions of articular surfaces, subcortical sclerosis, and cysts formation with iliac dominance. The right iliolumbar ligament calcification was also seen [Figure 2b].
MRI (1.5 Tesla GE Healthcare Signa HDXT) was also performed with axial, coronal, and sagittal images using T1-weighted (W) turbo spin-echo, T2-W turbo spin-echo sequences integrated with fat suppression, and contrast-enhanced T1-W sequences in multiple planes.
MRI revealed a T1 and T2 heterogeneous expansile mixed signal intensity enhancing lesion centered at the junction of the right pedicle and transverse process, as well as lamina of L5 vertebra, partly involving the contralateral left pedicle and lamina, extending into the spinal canal/epidural space, causing compression and left lateral displacement of the thecal sac with impingement of cauda equina nerve roots. The foraminal component of the lesion was compressing the right exiting L5 nerve roots. Confluent marrow edema and ill-defined but avid enhancement were noted in L5 and L4 vertebral bodies. Periostitis and enhancement were also noted in the body, bilateral pedicles, and spinous process of the L5 vertebra. Extensive right para/prevertebral and bilateral posterior paraspinal intramuscular and soft-tissue edema were noted showing strong enhancement. There was associated right L4-L5 and L5-S1 facet hypertrophic arthropathy with periarticular soft-tissue enhancement. The right psoas muscle was anteriorly elevated. L4-L5 and L5-S1 intervertebral discs were preserved [Figures 3a-d and 4a-c].
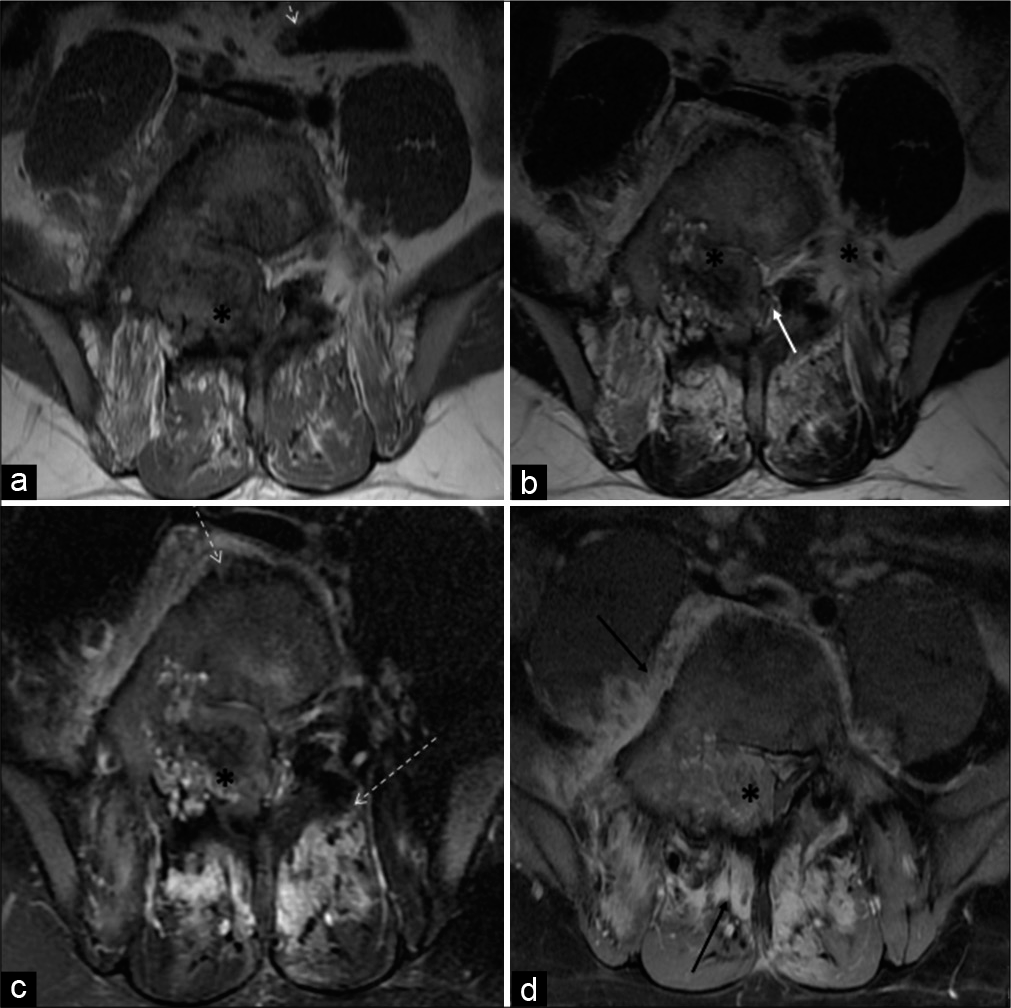
- A 23-year-old male who was diagnosed with osteoblastoma showing toxic flare and coexistent HLA-B27-related ankylosing spondylitis. Axial T1-W (a) and axial T2-W (b) MR images reveal heterogeneous mixed signal intensity expansile lesion (asterisk) centered in the right neural arch of the L5 vertebral body involving right pedicle, transverse process, and part of the lamina. It is extending into the spinal canal and causing compression/displacement of thecal sac (white arrow). Axial T2-W fat-suppressed (c) and axial T1-W fat-suppressed post-contrast MR images (d) reveal significant periostitis depicted along the vertebral body and contralateral posterior elements (dashed arrows). Heterogeneous and avid enhancement is seen in the lesion (asterisk) with enhancing pre, right paravertebral, posterior paraspinous soft tissues (black arrow).
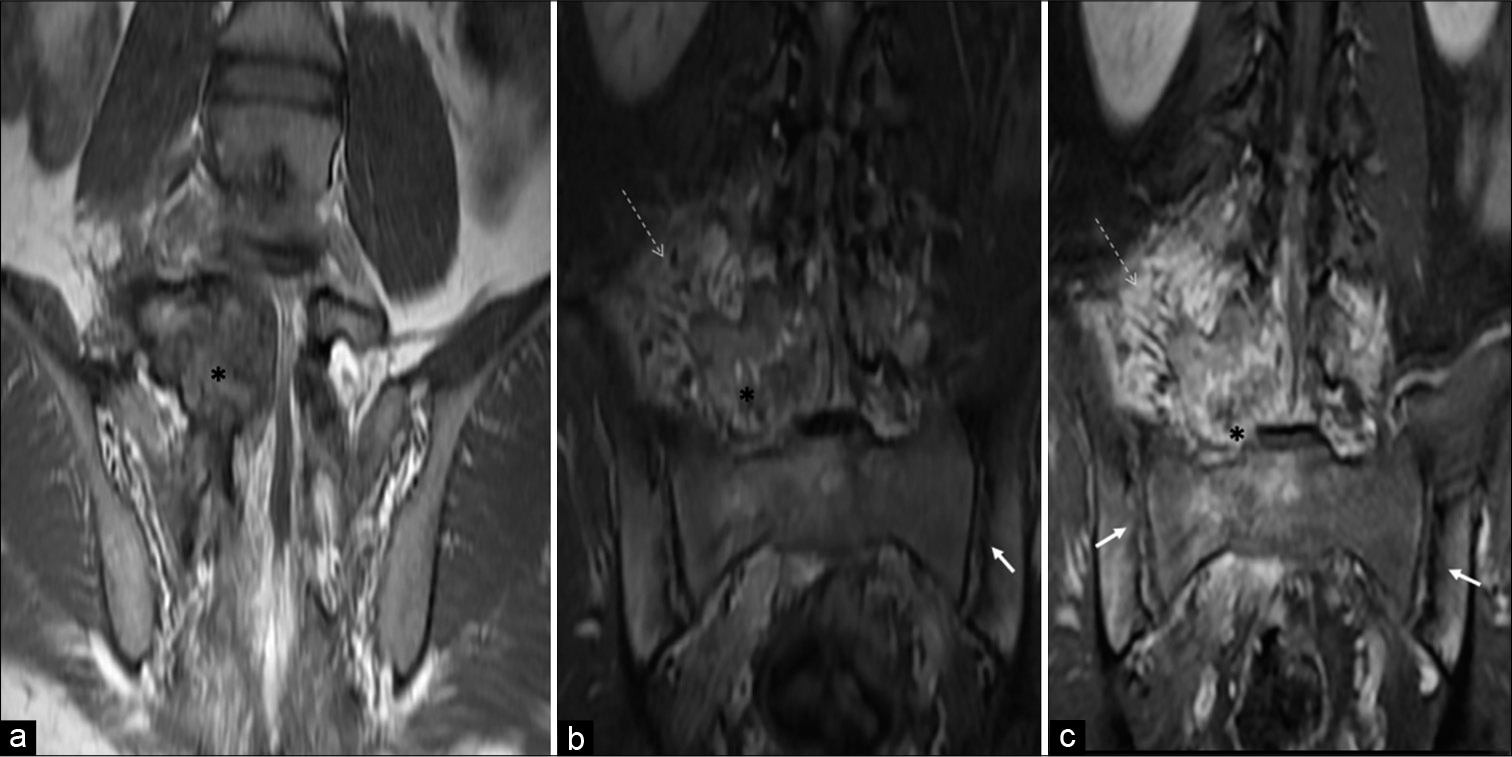
- A 23-year-old male who was diagnosed with osteoblastoma showing toxic flare and coexistent HLA-B27-related ankylosing spondylitis. Coronal T1-W (a), coronal short-tau inversion recovery (b), and coronal T1-W fat-suppressed post-contrast MR (c) images reveal osteoblastoma (asterisk) centered in the right neural arch of L5 vertebra showing heterogeneous enhancement with significant periostitis, STIR hyperintense, and enhancing right paravertebral/perilesional soft-tissue edema (dashed arrows) associated with coexisting bilateral symmetrical inflammatory acute sacroiliitis (arrows).
Besides this, changes of bilateral symmetrical acute sacroiliitis were seen in the form of irregularity and erosions of articular surfaces, subchondral edema, and cysts formation – more on the iliac side. Abnormal enhancement was seen in subchondral marrow, bilateral sacroiliac joints capsule, and juxta-articular deep soft tissues. There was no significant joint effusion or periarticular collection [Figure 4b and c].
Based on the above-described imaging findings, the diagnosis of AS with aggressive osteoblastoma showing a local flare phenomenon was made. Low-grade osteosarcoma was kept as close imaging differential as there has been an increased risk of bone cancers in patients with HLA-B27-positive AS.
The patient underwent CT-guided biopsy and histopathology revealed osteoblastoma [Figure 5].
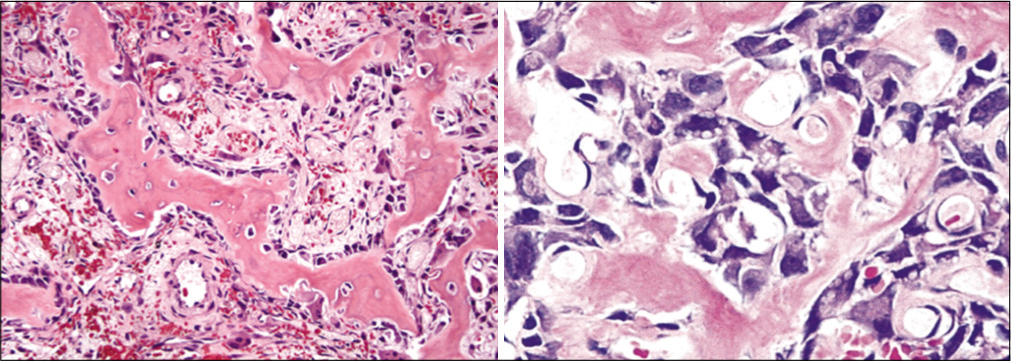
- Histopathological examination showed anastomosing trabeculae of woven bone lined by a single layer of plump osteoblasts within a loosely textured fibrovascular stroma suggesting osteoblastoma.
DISCUSSION
Osteoblastomas are benign tumors comprising osteoblasts producing osteoid and bone. There is a striking similarity in the histology of osteoid osteoma and osteoblastoma; however, they are distinguished by size, imaging features, and clinical manifestations. Osteoblastomas are predominantly seen in the axial skeleton, and one-third of the cases are seen in the spine, rest seen in decreasing order, in the long bones of the limbs, pelvis, and foot. They may also be observed infrequently in the cranial and facial bones.[1]
Osteoblastoma accounts for 1–3% of all primary bone tumors, with a male-female ratio of 2:1 and usually seen in the second to third decades. The most frequent site in the vertebral column is the sacrum involving the posterior elements.[2] Patients who present with toxic flare phenomenon are much younger, ranging from 5 to 12 years.[5]
Osteoblastomas have the potential for progressive growth and may sometimes pursue an aggressive clinical and radiological course. Patients usually present with low backache, rigidity, and stiffness, which is not relieved with aspirin. On immunohistochemical examination, cyclooxygenase (COX)-1 and COX-2 positivity are seen in patients with toxic flare, indicating that the tumor activates the arachidonic acid metabolic pathway producing prostaglandin.[4,6,7] Aggressive behavior is within the biological spectrum of osteoblastoma, and the most important differential diagnosis is osteosarcoma.[3,5]
On plain radiographs, osteoblastomas are more than 2 cm in size, expansile, usually affecting the neural arch, with a clearly defined, thin cortical rim at the periphery. Reactive sclerosis adjacent to the lesion is common at conventional radiography.[2,3]
CT generally offers detailed information in addition to plain radiography. Tumor extent, its delineation, and matrix mineralization are better depicted on CT scans. The lesion matrix is usually lytic; however, mixed blastic-lytic changes can occasionally occur. Widespread, ill-defined sclerotic bone formation around the lesion and periosteal reaction/ periostitis can be seen. There is usually high intralesional contrast uptake due to rich vascularity. Aggressive osteoblastoma with cortical breakthrough and a broad zone of transition is better delineated on CT.[2,5,8]
MRI is useful for assessing the involvement of adjacent structures from the bony expansion. It helps to determine the extension of vertebral lesions into the spinal canal and spinal foramina. Lesions show iso to intermediate signal intensities on T1-W images. T2-W images depict hypo to hyperintense tumor signals, depending on the degree of tumor matrix mineralization.[2,5,7]
Variable enhancement of the tumor matrix is noted on post-contrast sequences. Peritumoral edema is commonly seen involving adjacent bones and soft tissues, showing a variable degree of enhancement. In some cases, flare phenomenon is seen, which is defined as inflammatory soft-tissue reaction presenting as marrow edema, periosteal reaction, and periostitis. This unusual phenomenon is likely due to the interleukins-mediated immune response mounted by the patient to the tumor.[3,4,6]
Post-contrast enhancement depicting the inflammatory nature obscures the actual lesion leading to suboptimal interpretation and characterization on MR images: Hence, the tumor’s appearance is sometimes misleading on MR due to variable appearance, adjacent inflammatory changes, reactive bone, and soft-tissue changes.[5,8]
Low-grade osteosarcoma, Ewing’s sarcoma, osteomyelitis, and lymphoma are close imaging differentials in patients presenting with severe flare phenomenon.[2,7,8]
Literature depicting the flare phenomenon with the systemic presentation in spinal osteoblastoma is sparse. Crim et al. in 1990[4] published a case of vertebral osteoblastoma, causing a diffuse, reactive inflammation in two vertebrae, adjacent ribs, and the paraspinous soft tissues. However, remote multifocal systemic manifestations were not seen.
Mirra et al. in 1979[6] published a case of osteoblastoma of the proximal femur in a 7-year-old with massive reactive periostitis mimicking osteosarcoma or osteomyelitis and severe systemic manifestations of fever, leukocytosis associated with multifocal symmetrical periostitis at the diaphysis of long bones, and weight loss.
Dale in 2001[3] reported a similar case of toxic flare with severe systemic manifestations in a 5-year-old boy, presenting with a large growing left arm mass, low-grade fever, and weight loss. Extensive periosteal reaction was seen along the shafts of the humerus, radius, ulna, and metacarpals. Long bones of lower extremities also showed diffuse acute periostitis bilaterally.
The current case is similar to the case published by Crim et al. in 1990.[4] Our patient presented with similar symptoms of intermittent fever, pain, stiffness, and imaging revealed an expansile lesion centered in the right neural arch of L5 vertebra extending into the vertebral body with marrow edema, extraosseous soft-tissue component, features of repeated periostitis, sclerosis extending up to L4 vertebral body, and confluent peritumoral edema, representing extensive flare phenomenon. There was coexistent acute inflammatory sacroiliitis – AS related to HLA-B27 positivity.
As we know, AS is a complex immune-mediated condition. Genetic factors influencing antigen presentation and the interleukins IL-23/17 A pathway confer a predisposition to AS. AS, combined with several other comorbidities, is a risk factor for cancer development in males and females.[9]
Heightened inflammatory/flare phenomenon as seen in our case can be partly explained by immune inflammatory axis activation in AS with activation of Th17 or other CD4+ cells, mast cells, neutrophils, and other innate immune cells resulting in the production of IL-17A, IL-22, TNF-a, IFN-g, and other cytokines even though the definite association of the flare phenomenon with HLA-B27 is unknown.[9,10]
CONCLUSION
Osteoblastomas are bone-forming neoplasms with a predilection for the axial skeleton. CT is the modality for the diagnosis of bone tumors that are poorly seen on conventional radiographs. MRI is very valuable in the detection of tumor extension and in differentiating soft-tissue tumors from reactive edema. This case is unusual in terms of the association of HLA-B27 – AS with the coexisting local toxic flare phenomenon of osteoblastoma, which by itself is infrequently noted. The incidence of osteosarcoma in a young adult with AS is known in few studies, however, osteoblastomas and their association with AS are not yet established.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Benign bone-forming lesions: Osteoma, osteoid osteoma, and osteoblastoma, Clinical imaging, pathologic, and differential considerations. Skeletal Radiol. 1993;22:485-500.
- [CrossRef] [Google Scholar]
- Diagnostic imaging of solitary tumors of the spine: What to do and say. Radiographics. 2008;28:1019-41.
- [CrossRef] [PubMed] [Google Scholar]
- Severe toxic osteoblastoma of the humerus associated with diffuse periostitis of multiple bones. Skeletal Radiol. 2001;30:464-8.
- [CrossRef] [PubMed] [Google Scholar]
- Widespread inflammatory response to osteoblastoma: The flare phenomenon. Radiology. 1990;177:835-6.
- [CrossRef] [PubMed] [Google Scholar]
- CT and MR imaging of vertebral osteoblastoma. A report of two cases. Clin Imaging. 1996;20:37-41.
- [CrossRef] [Google Scholar]
- A case of osteoblastoma associated with severe systemic toxicity. Am J Surg Pathol. 1979;3:463-71.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging algorithm and multimodality evaluation of spinal osteoblastoma. BMC Musculoskelet Disord. 2020;21:240.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal osteoblastoma: CT and MR imaging with pathological correlation. Skeletal Radiol. 1999;28:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Ankylosing spondylitis and the risk of cancer. Oncol Lett. 2017;14:1315-22.
- [CrossRef] [PubMed] [Google Scholar]
- Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology (Oxford). 2018;57(Suppl 6):vi4-9.
- [CrossRef] [PubMed] [Google Scholar]






