Translate this page into:
Ultrasound and computed tomography-guided interventions for the hip joint and pelvis: Comprehensive imaging review

*Corresponding author: Vikas Batra, Department of Radiology, Fortis Escorts Hospital, Faridabad, Haryana, India. drvikas28@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Batra V, Spalkit S, Bugata S, Chari R, Batta NS. Ultrasound and computed tomography-guided interventions for the hip joint and pelvis: Comprehensive imaging review. Indian J Musculoskelet Radiol. 2025;7:108-18. doi: 10.25259/IJMSR_4_2025
Abstract
Diagnosing the underlying cause of hip pain can be challenging, often requiring extensive clinical and imaging workup. Current definitive treatments, while effective, frequently involve invasive surgical procedures with inherent risks, potential complications, and the possibility of complex revision surgeries. For radiologists, ultrasound (US)- and computed tomography (CT)-guided musculoskeletal interventions offer compelling, minimally invasive, and cost-effective alternatives. These image-guided techniques provide precise, real-time visualization, enabling accurate diagnoses and targeted therapies. This review comprehensively examines various US- and CT-guided techniques for managing hip pain, including anesthetic and corticosteroid injections for pain relief and inflammation reduction, viscosupplementation for joint lubrication and cartilage health, and platelet-rich plasma therapy for tissue regeneration and healing. The review also details the relevant anatomy of key structures frequently implicated in hip pain, encompassing periarticular structures, bursae (such as the iliopsoas, greater trochanteric, and ischiogluteal bursae), nerves (including the ilioinguinal and lateral femoral cutaneous nerves), and muscles (such as the piriformis and quadratus femoris). By mastering these advanced techniques and acquiring a thorough understanding of the relevant anatomy, interventional radiologists can play a pivotal role in optimizing the evaluation and management of hip pain, offering patients more precise diagnoses and less invasive treatment options, ultimately leading to improved patient outcomes and reduced healthcare costs.
Keywords
Hip and Pelvic pain
Image-guided interventions
Interventional radiology
Nerve blocks
Ultrasound - computed tomography
INTRODUCTION
Hip pain, a pervasive and increasingly prevalent condition, poses significant diagnostic and therapeutic challenges. While hip osteoarthritis is a common culprit, a myriad of other musculoskeletal conditions can contribute to hip pain, affecting not only the joint itself but also adjacent structures such as tendons, muscles, bursae, and peripheral nerves.
The complex anatomy of the hip region often complicates the accurate identification and effective management of the underlying causes.
In this context, image-guided injections, utilizing ultrasound (US) and computed tomography (CT), have become indispensable tools for both diagnostic clarification and therapeutic intervention. These minimally invasive procedures offer targeted access to a variety of superficial and deep structures around the hip, providing valuable solutions for managing pain regardless of the specific technical approach, imaging modality, or medication used.
Image-guided injections can be instrumental in refining the differential diagnosis by pinpointing pain sources, serving as a primary treatment modality for managing pain, and offering a temporary bridge to surgery when indicated.
This comprehensive review explores both ultrasound (US)- and CT-guided procedures for the hip, providing a concise overview of relevant medications, core principles of image-guided interventions, and specific injection techniques for common hip pathologies.
Before delving into the specifics of each intervention, we will first outline the basic steps common to all image-guided procedures [Table 1].
| Checklist Item | |
|---|---|
| Patient preparation | Confirm no contraindications |
| Ensure patient understands the procedure and risks | |
| Review clinical data and ongoing treatment | |
| Check for allergies and medications | |
| Confirm fasting requirements | |
| Adjust anticoagulants if needed | |
| Imaging preparation | Ensure ultrasound/CT machines are functional |
| Conduct ultrasound/CT to plan needle path Strict aseptic precautions for site preparation and optimal patient positioning | |
| Use sterile gel or chlorhexidine. | |
| Inject local anaesthetic under guidance | |
| Staff coordination: | Ensure team members understand their responsibilities with clear communication protocols |
| Emergency preparedness | Ensure resuscitation tools are available |
| Familiarize with emergency protocols | |
| Post procedure care | Use pressure on the entry site and sterile dressing cover |
| Documentation | Complete checklist, vital signs and relevant information |
BASIC PRINCIPLES OF IMAGING-GUIDED INTERVENTIONS
Key medications
Local anesthetics
Local anesthetics are widely utilized for immediate pain relief and to provide diagnostic insight in identifying sources of pain.[1]
Intra-articular and intravascular administration of these anesthetics in large quantities should be avoided, as serious central nervous system and cardiovascular adverse effects have been reported [Table 2].
| Anesthetic | Onset (minutes) | Duration of anesthesia (hours) | Duration of analgesia (hours) | Maximum dose (mg/kg) without/with epinephrine |
|---|---|---|---|---|
| 2% LIDOCAINE | 10 to 20 | 2 to 5 | 3 to 8 | 4.5/7 |
| 1.5% MEPIVACAINE | 10 to 20 | 2 to 5 | 3 to 10 | 5/7 |
| 0.2% ROPIVACAINE | 15 to 30 | NA | 5 to 16 | 3/3.5 |
| 0.5% ROPIVACAINE | 15 to 30 | 4 to 12 | 5 to 24 | 3/3.5 |
| 0.25% BUPIVACAINE | 15 to 30 | Na | 5 to 26 | 2.5/3 |
Corticosteroids
Corticosteroids are widely used for their anti-inflammatory properties, offering medium-term relief from symptoms. Commonly used long-acting corticosteroids for intraarticular injections include triamcinolone acetonide and methylprednisolone acetate.[1-3]
Side effects, contraindications, and recommendations of corticosteroids is shown in Tables 3-5.
| Tendon damage | Avoid intra and repeated peritendinous injections |
| Cartilage breakdown | |
| Post-Injection Flare | Local inflammatory flare-up in 2–25% of cases, lasting up to 3 days |
| Skin and subcutaneous side effects | Skin atrophy, fat necrosis, depigmentation; Methylprednisolone preferred for injections near skin |
| Impact on blood glucose levels | Transient increase in blood glucose levels in 1% of patients, 2–5 days after injection |
| Facial flushing | More common with triamcinolone; diphenhydramine recommended if ersistent |
| Absolute contraindications |
| • Local or intraarticular sepsis • Bacteraemia • Known hypersensitivity to injection components. |
| Relative contraindications |
| • Severe juxta-articular osteopenia • Coagulopathy If INR is greater than 2 • More than three intraarticular injections within the same year • A previous injection within the last 6 weeks |
| Recommendations |
|---|
| • Avoid intraarticular injections unless necessary, such as in end-stage osteoarthritis. • Rest for 2 weeks post-injection and avoid heavy loading for 6 weeks. • Allow 6 weeks between injections to assess effects. • Limit to three injections per site per year. • Do not repeat if less than 4 weeks of relief is achieved after two injections. |
Platelet-rich plasma (PRP)
PRP therapy is increasingly used to treat musculoskeletal injuries. It utilizes platelets to release growth factors that support tissue healing, especially in low-blood-supply areas like tendons, offering longer-lasting relief compared to corticosteroids.
Before administering PRP injections, patients are thoroughly educated about the procedure, including its potential benefits and risks, such as infection, hemorrhage, and soft-tissue injury. To mitigate these risks, strict sterile techniques are employed. PRP is contraindicated in cases of local infection, inflammation, or a history of malignancy. Patients are also advised to avoid non-steroidal anti-inflammatory drugs (NSAIDs) for 2 weeks before and after the procedure to preserve the activity of growth factors. Post-procedural pain is expected due to the induced inflammatory response, and physical therapy typically commences 2 weeks after the injection.
The preparation of PRP involves collecting autologous blood into a syringe containing an anticoagulant. Centrifugation separates the blood into three components: Red blood cells (RBCs), platelet-poor plasma, and a platelet concentrate containing white blood cells. The RBC layer is discarded, and a secondary centrifugation step further concentrates the platelets. Approximately 3 mL of PRP can be obtained from 30 mL of blood following a 15-min centrifugation at 3,200 revolutions per minute. PRP typically achieves a platelet concentration 5 times higher than normal (1,000,000/μL) and is prepared under sterile conditions for immediate use.
Administration is performed using a 20- or 22-gauge needle under US guidance. Needle-induced bleeding activates platelets through thrombin. The use of local anesthetics is generally avoided due to uncertain effects on PRP efficacy.
Standardization remains challenging, and the comparative efficacy of different preparation methods and platelet concentrations has not been conclusively established.[4,5]
Diagnostic interventions
These Image-guided CT and USG interventions can be divided into:
Diagnostic interventions.
Therapeutic interventions.
CT-guided bone biopsies
Radiologists play a crucial role in diagnosing patients undergoing limb-sparing surgery, particularly through image-guided needle biopsies. These biopsies offer similar diagnostic accuracy to open biopsies while reducing complications and costs. However, bone sarcomas pose a risk of local recurrence due to tumor cell seeding along the biopsy tract, especially with larger-gauge needles. Therefore, radiologists should treat all solitary bone lesions as potential sarcomas and collaborate with orthopedic oncologists to plan biopsy entry points strategically. Pre-operative planning is essential to align biopsy tracts with surgical incisions, ensuring the entire tract can be excised during surgery to minimize recurrence risk. This meticulous coordination helps preserve critical structures, optimizing surgical outcomes and functional limb preservation. For lesions in the proximal femoral head and neck, biopsies should align with the standard surgical approach involving a longitudinal incision along the lateral thigh. This approach facilitates complete excision of the biopsy tract during the definitive surgical procedure[6,7] [Figure 1].
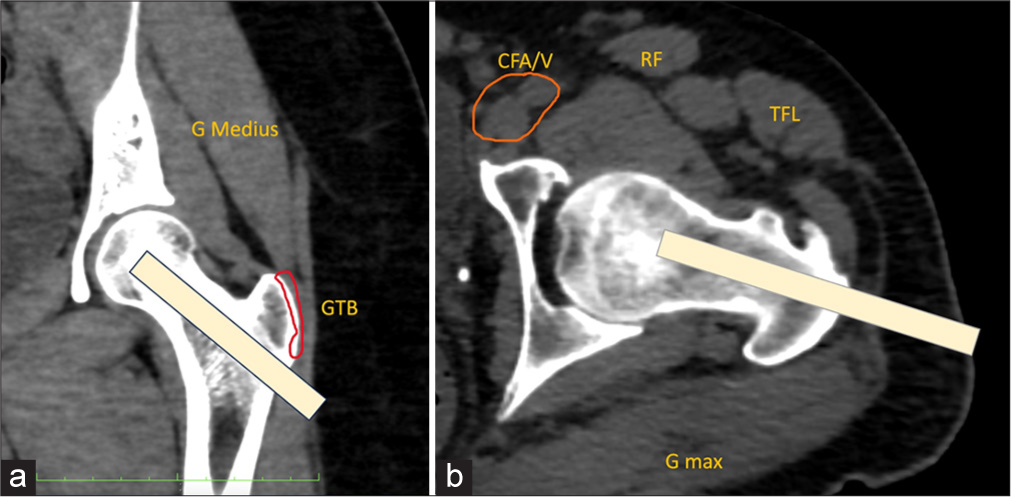
- (a) Coronal and (b) axial oblique multi-planar reformatted computed tomography images show a normal left hip. An angled subtrochanteric approach (yellow strip in a and b) should be used for the biopsy of lesions in the femoral neck or head. The greater trochanter bursa (outlined in red in a) and the hip joint capsule should be avoided. GMax: Gluteus maximus muscle, GMedius: Gluteus medius muscle, TFL: Tensor fascia lata muscle, orange outlined is CFA/V: Common femoral artery and vein.
Essential structures that must be avoided are:
Greater trochanteric bursa
Femoral neurovascular bundle
Transverse branch of the lateral femoral circumflex artery
Rectus femoris muscle.
Recommended biopsy approach
Begin at the inferolateral aspect of the hip in the subtrochanteric region. Direct the needle superomedially through the femoral neck, following an intraosseous trajectory to prevent contamination of the joint capsule.
For cystic lesions, perform an aspiration biopsy
For solid, non-sclerotic lesions (>50% soft tissue), use a Jamshidi needle
For sclerotic lesions (>50% bone), use large needles (7–14 gauge); drilling may be necessary for thick cortices.[8]
CT-guided biopsy and aspirations of the sacroiliac joint
CT-guided aspiration and biopsy of the sacroiliac joint are indicated for:
Suspected Infection
Inflammatory disorders such as ankylosing spondylitis or inflammatory arthritis
Tumors, both primary and metastatic
Chronic pain with unclear causes, to provide diagnostic information.[9]
Technique
The patient lies prone. Pre-procedural steps, including a CT scan, determine the needle’s optimal entry point. For aspiration, fluid is drawn from the joint for laboratory analysis. For a biopsy, tissue or cartilage samples are taken [Figure 2]. Afterward, the needle is removed, pressure is applied to prevent bleeding, and the patient is monitored for complications.
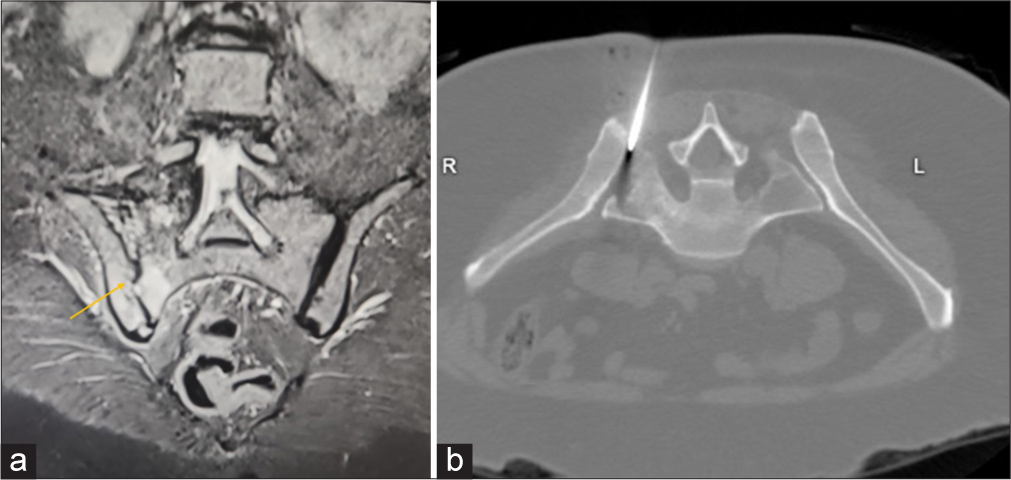
- (a) Coronal Short tau inversion recovery (STIR) sequence image depicts acute inflammatory sacroiliitis orange arrow of the right sacroiliac joint. (b) Axial computed tomography image demonstrates the final needle trajectory placed in right sacroilliac joint for aspiration/biopsy.
CT/USG-guided hip joint synovial biopsy/aspiration
It is a minimally invasive procedure used to diagnose various joint conditions, such as infections and inflammatory diseases.
Synovial biopsy
To investigate septic versus inflammatory arthritis.
Synovial aspiration
To remove excess effusion caused by inflammation or infection.
US is commonly used due to its cost-effectiveness, technical simplicity, patient acceptability, and high synovial sampling success rate.[2,10] US Gray Scale and Power Doppler are used as guides to target the area of highest inflammation. Multiple synovial biopsies are taken from different parts of the joint.
Technique
The patient is placed in the supine position. The needle is inserted in an in-plane caudal-to-cranial approach, with the convex probe parallel to the femoral neck, targeting the head-neck junction. Color Doppler aids in visualizing the injectate’s intra-articular diffusion [Figure 3].
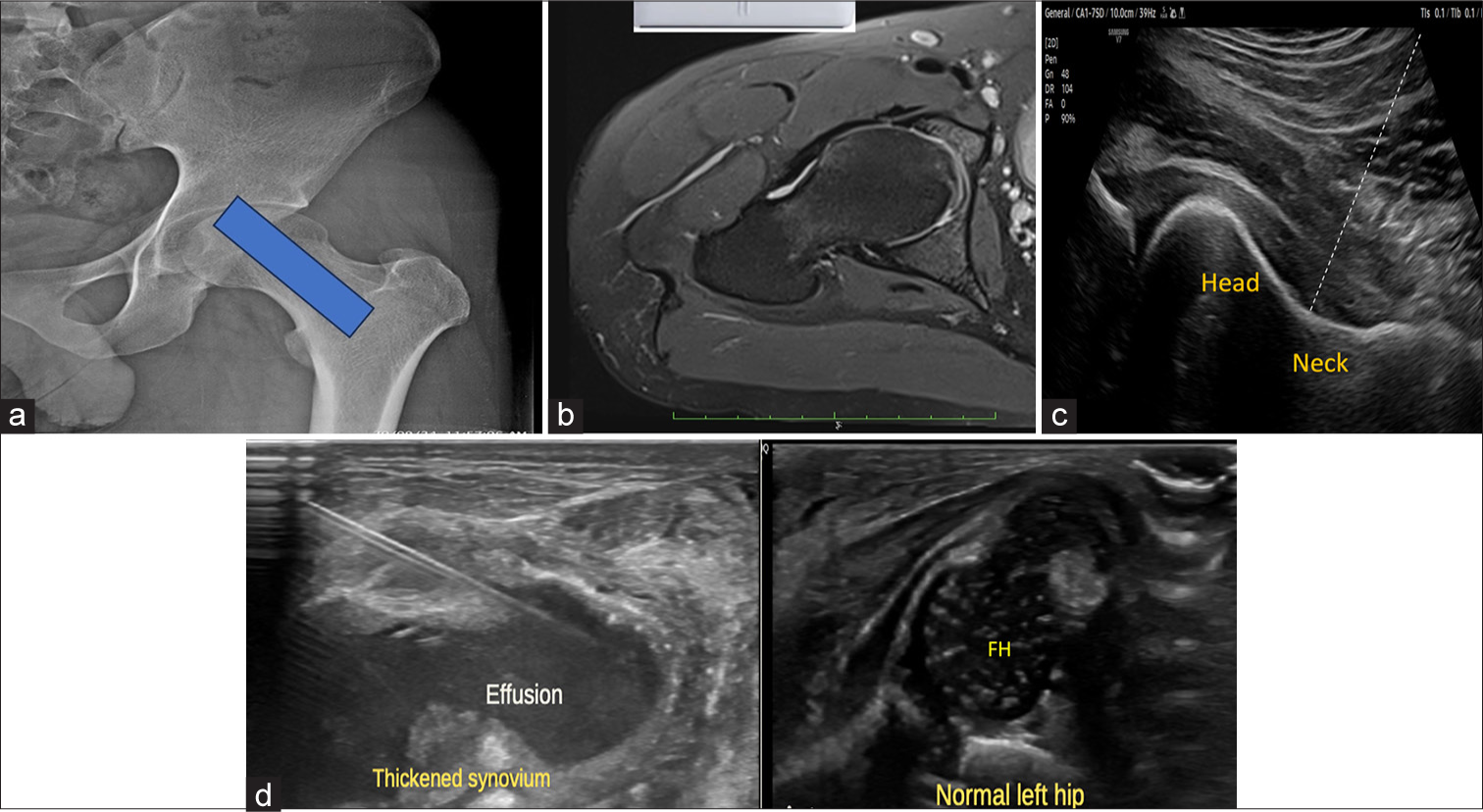
- (a) Anteroposterior hip radiograph with overlying blue box shows the site/positioning of the ultrasound probe parallel to the long axis of the femoral neck. (b) Corresponding axial proton density fat saturated magnetic resonance image demonstrates probe site for needle position. (c) Ultrasound at the corresponding level reveals the femoral head junction, the site for needle insertion. The dotted line demonstrates the needle trajectory. (d) Companion case of a 13-day-old neonate presented with swelling in the right hip. Local examination revealed a swollen and tender right hip. Ultrasonography revealed a significant amount of fluid with internal echoes in the right hip joint with synovial thickening. Under aseptic precautions, 12 mL of thick pus was aspirated. Ultrasound images of the affected right hip reveal joint effusion with internal echoes and thickened synovium. The needle track is visualized. Ultrasound image of normal left hip demonstrates normal femoral head capital epiphyses. FH: Femoral head.
Therapeutic interventions
Anterior HIP
For degenerative osteoarthritis
US and fluoroscopy are favored modalities in cases of therapeutic interventions. Hyaluronic acid injections are indicated for hip osteoarthritis patients who cannot manage pain with NSAIDs or corticosteroids or have contraindications.[1,11]
Hyaluronic acid injections protocol: One injection weekly for 3–5 weeks. Benefits peak around 5 weeks post-injection and last up to 3 months.[12] A pseudoseptic reaction may occur post-injection but is manageable conservatively.
Ilioinguinal and genitofemoral nerve block
The ilioinguinal (L1) and iliohypogastric (T12 and L1) nerves, branches of the lumbar plexus, course through the transversus abdominis and lie between the internal oblique and transversus abdominis muscles. Together with the genitofemoral nerve, these “border nerves” supply the skin at the junction of the abdomen and thigh[13,14] [Figure 4a].
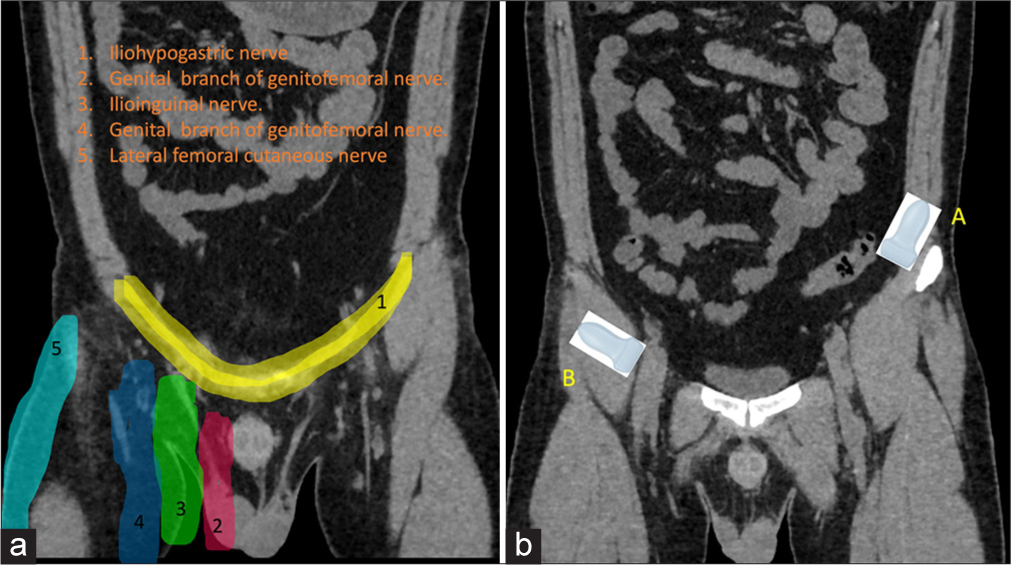
- (a) Dermatomal distribution of pelvis. (b) Position of the ultrasound probes for A: Ilioinguinal and B: genitofemoral nerve blocks.
Indications: These nerve blocks are effective for postoperative pain relief following procedures such as inguinal hernia repair, orchiopexy, lower abdominal surgeries (Pfannenstiel incisions and appendectomies), and laparoscopic surgeries.[1]
-
Technique for ilioinguinal block: The patient is placed in the supine position. The probe is placed over the bony prominence of the anterior superior iliac spine (ASIS) and the other side points toward the umbilicus. The neurovascular bundle is identified and located between the internal oblique and transversus abdominis muscles. The nerves are visualized just above and medial to the shadow of the iliac crest or ASIS [Figure 4b]. The block is performed as laterally as possible to avoid nerve branching anteromedially [Video 1]. Insert the needle from medial to lateral [Figure 5].
Video 1:
Video 1:Video shows a normal neurovascular bundle of ilioinguinal nerve in the transverse abdominis plane.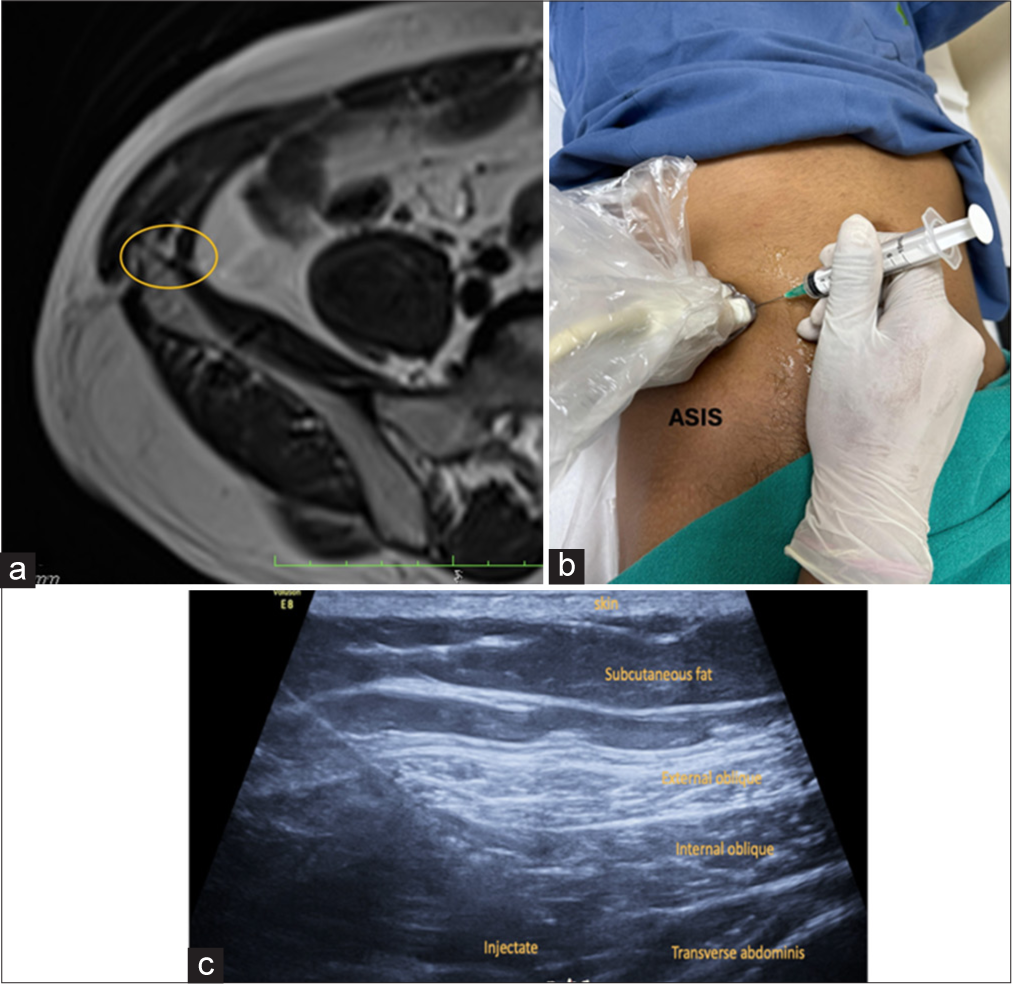 Figure 5:
Figure 5:- (a) Axial T1-weighted magnetic resonance image depicts the normal site marked by yellow circle of the ilioinguinal nerve within the transverse abdominis plane in the anterolateral abdominal wall. (b) The patient is in the supine position for the block with probe positioned superomedial to the ASIS. ASIS: Anterior superior iliac spine (c) ultrasound image shows final injectate in the transverse abdominis plane.
Injectate: 5–7 mL mixture comprising 2% lidocaine mixed with 2 mL of dexamethasone.
-
Technique for genitofemoral block[15]
Position the patient supine
Place a linear US transducer over the femoral artery and move cephalad where it transitions to the external iliac artery
Identify the inguinal canal/spermatic cord in men and the round ligament in women
Position one finger breadth from the pubic tubercle and identify the testicular artery in males
Use an in-plane or out-of-plane technique
Injectate: Men: Inject 4 mL lidocaine 2% with 1 mL dexamethasone within and 4 mL outside the spermatic cord [Figure 6]
Women: Inject 5 mL of local anesthetic around the round ligament
Use plain local anesthetic without epinephrine to avoid vasoconstriction of testicular arteries.
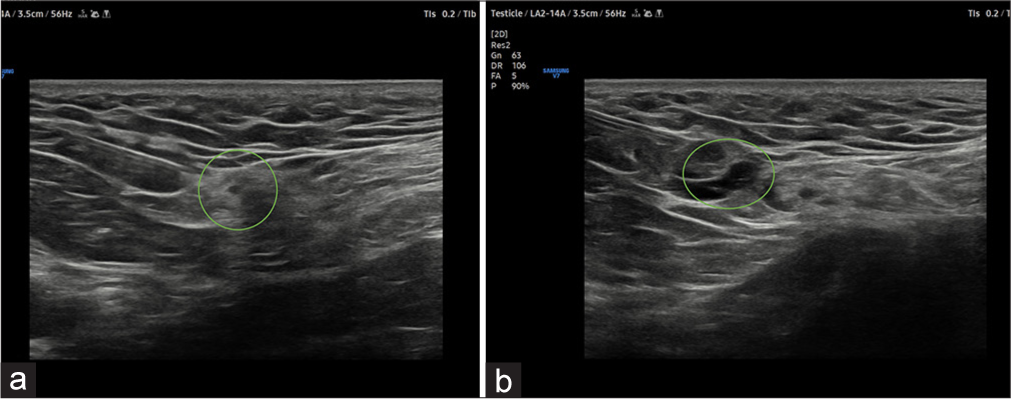
- (a) USG image shows the needle trajectory from the lateral to the medial approach for genitofemoral nerve block. Green circle depicting the spermatic cord structures. (b) There is the spread of injectate green circle surrounding the genitofemoral nerve.
Meralgia paresthetica: Lateral femoral cutaneous nerve (LFCN)
Meralgia paresthetica is used to describe the altered sensation in the anterolateral thigh caused by LFCN neuropathy, sensory nerve arising from posterior divisions of L2 and L3 nerve roots. In the proximal thigh, it runs in the superficial fascial plane between the sartorius and tensor fascia lata muscles, which are landmarks for nerve blocks.
The nerve is most affected at the level of the inguinal ligament due to mechanical compression, due to external factors such as tight clothing, seat belts or internal factors – obesity or pregnancy.[1,2,11]
Technique
The patient is positioned supine, and a linear probe is placed transversely at the level of the ASIS. The nerve is visualized between the medial portion of the tensor fasciae lata muscle and the sartorius muscle. A 22-gauge needle is inserted using an in-plane approach from lateral to medial to access the LFCN, located beneath the inguinal ligament and superficial to the sartorius muscle [Figure 7].
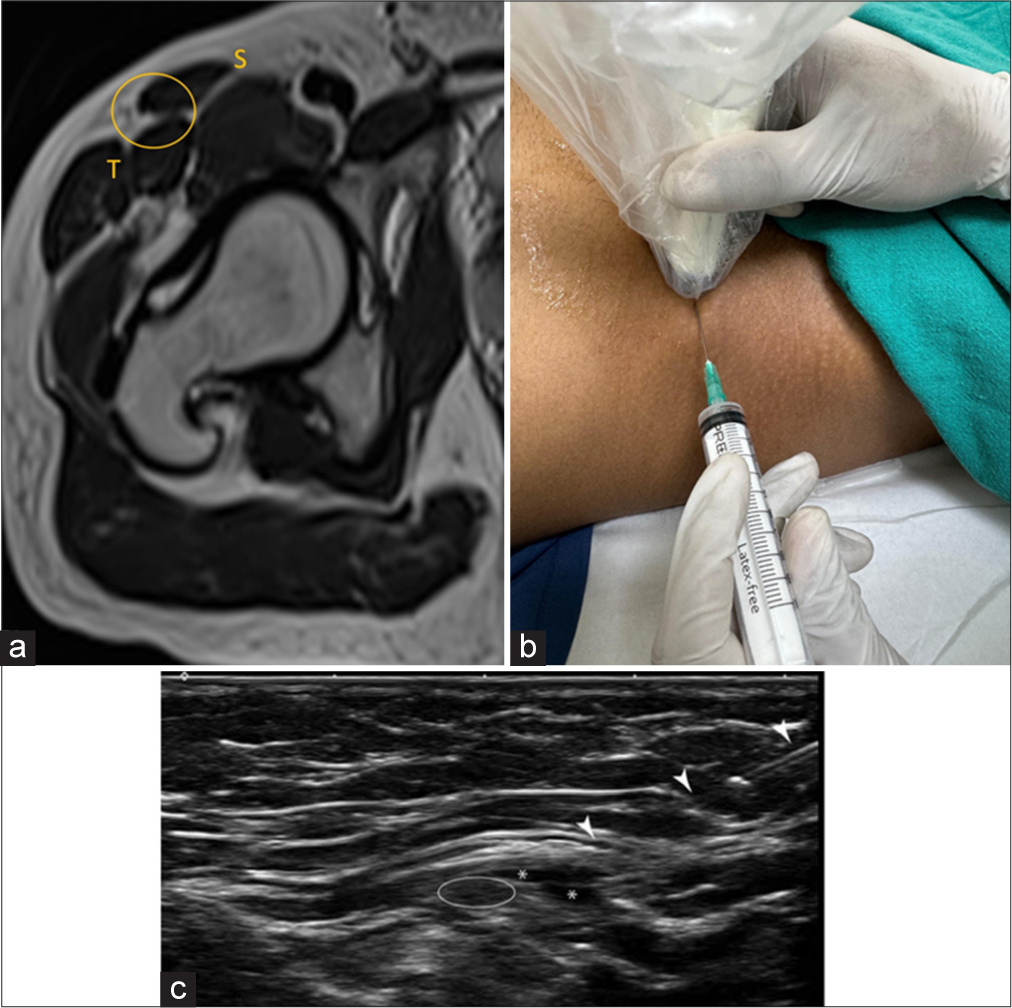
- (a) Axial T2-weighted magnetic resonance image image at the level of right hip depicts the normal plane of the lateral femoral cutaneous nerve and site of injection marked by yellow circle. S: Sartorius and T: tensor fascia lata. (b) The patient is in a supine position with transducer placed at anterosuperior iliac spine. The needle is inserted from the lateral to medial in-plane approach. (c) USG image at the corresponding level demonstrates needle track (arrowhead) and white circle demonstrating lateral femoral cutaneous nerve. Asterisk demonstrates the spread of injectate.
Injectate
3–5 mL of total mixture comprising 2% lidocaine mixed with 10 mg of dexamethasone.
Iliopsoas bursa
Iliopsoas impingement is one of the common causes of pain in post-total hip arthroplasty. US is used for the treatment of iliopsoas bursitis and tendinopathy.[16,17]
Technique
The patient is positioned supine and the transducer is placed parallel to the inguinal ligament. By positioning the probe transversely the iliopsoas tendon is identified at the level of acetabulum.
An in-plane lateral-to-medial approach is employed with a 22 G × 9 cm spinal needle
Positioning the needle is positioned laterally and deep to the iliopsoas tendon at the anticipated location of the bursa [Figure 8].
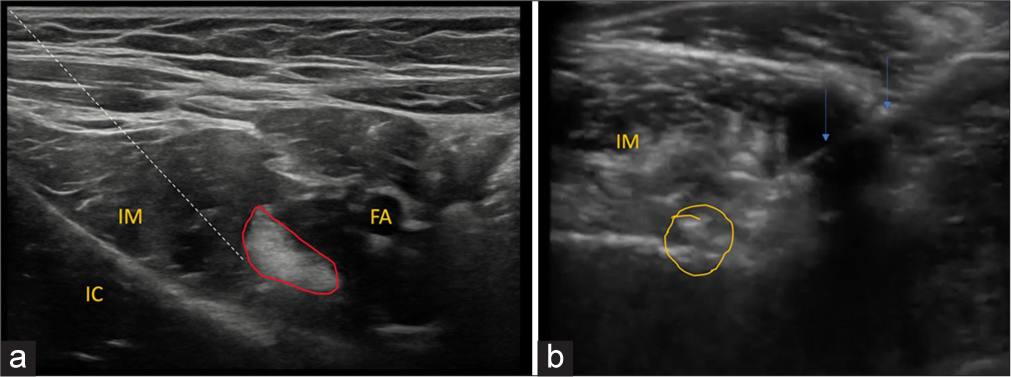
- (a) Ultrasound image at the level of iliopsoas myotendinous unit. The dotted line demonstrates the needle track. Iliopsoas myotendinous unit demonstrated in red outline. FA: Femoral artery, IC: Iliac crest, IM: iliacus muscle. (b) Axial ultrasound image demonstrates needle track-arrow within the iliopsoas bursa with the spread of injectate with few air loculi- circled in yellow deep to iliacus muscle.
Bursa visualization
The injection is targeted deep to the tendon when injecting the collapsed bursa to achieve proper visualization.
Injectate
Total volume of 8–10 mL comprising 2% lidocaine and 20 mg of methylprednisolone.
Lateral hip
Greater trochanteric bursitis
Greater trochanteric pain syndrome is characterized by pain and tenderness on the lateral side of the greater trochanter due to inflammation/bursitis of the greater trochanteric bursa encompassing the posterior facet.[1,2,11] The trochanteric region is a common site of pain in patients after total hip replacement, often resulting from insufficient post-operative rehabilitation, leading to weakness in the gluteal muscles.
Technique
The patient lies in a lateral decubitus position with the affected hip on top. The US transducer is placed over the greater trochanter in the short axis. A 22-gauge spinal needle is advanced in-plane from the posterior to the anterior aspect of the greater trochanter, entering the greater trochanteric bursa, which is located above the gluteus medius tendon insertion [Figure 9].
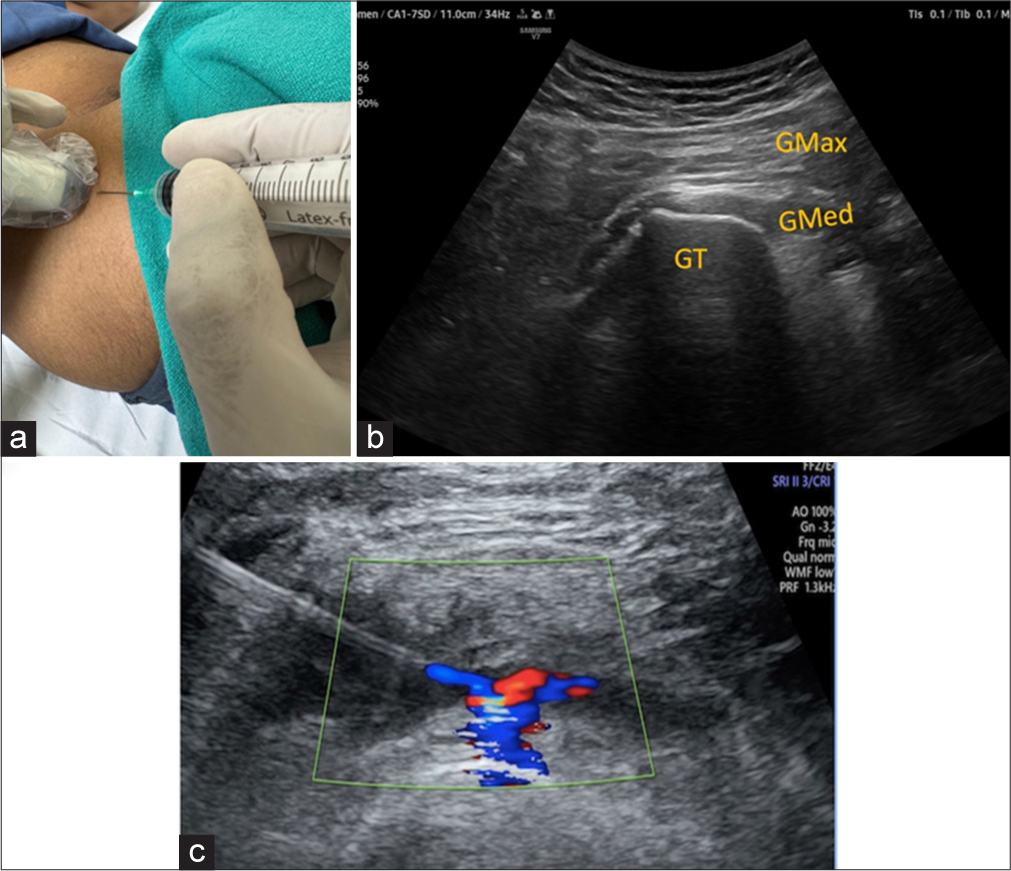
- (a) The patient is in a lateral decubitus position with an affected hip up. (b) USG image at the corresponding level demonstrates gluteus medius tendon inserting over GT. GT: Greater trochanter, Gmax: Gluteus maximus and Gmed: Gluteus Medius. (c) USG image with color Doppler images depicts the final spread of injectate. Green box demonstrates color doppler window..
Injectate
4 mL of 2% lignocaine plus 1 mL of depomedrone deposited in the bursa.
Posterior hip
Pudendal nerve block
It is used to diagnose and treat pudendal neuralgia, a condition characterized by chronic pelvic pain in the areas supplied by the pudendal nerve.[18]
Technique: The patient is in the prone position, and the probe is placed at the level of the ischial spine. The pudendal nerve is located medially, positioned between the pudendal artery (using Doppler) and the sacrotuberous ligament. The needle is inserted using an in-plane lateral-to-medial approach to access the pudendal nerve.
-
CT guidance: The target site is the pudendal canal [Figure 10].
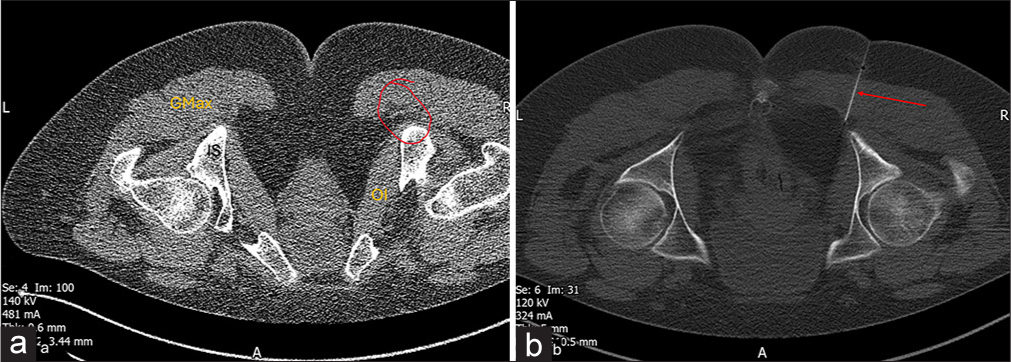 Figure 10:
Figure 10:- Post total hip arthroplasty status. Patient is in prone position. (a) Axial computed tomography (CT) image at the level of pelvis demonstrates planning with a grid attached to the skin surface. (b) Axial CT image shows the final spread of injectate for pudendal nerve block.
Landmarks: Caudad and medial to the ischial spine; medial to the obturator internus muscle; and into the pudendal canal, it is medially demarcated by the obturator internus aponeurosis.
Injectate: Total volume of 5–7 mL of 2% lidocaine and 1 mL of methyl prednisolone.
Hamstring tendinopathy
Hamstrings are the primary muscle groups consisting of Biceps femoris, semimembranosus and semitendinosus originating from the ischial tuberosity. Hamstring tendinopathy is a chronic overuse injury typically affecting the hamstring muscle origin. US findings reveal a thickened and hypoechoic tendon with hyperemia on Power Doppler.[19]
Treatment options: US -guided fenestration and PRP injection for hamstring tendinopathy are recommended when other non-invasive treatments have failed.[1]
-
Technique:
The patient is in the prone position
US transducer is in transverse orientation at the ischial tuberosity
Using an in-plane approach, the needle is advanced from lateral-to-medial direction. The Fenestration Technique is used to give five to ten passes.
Injectate: 3–4 mL of PRP is injected within the tendon. Most hypoechoic and hyperemic areas of the tendon origin and enthesis are targeted [Figure 11].
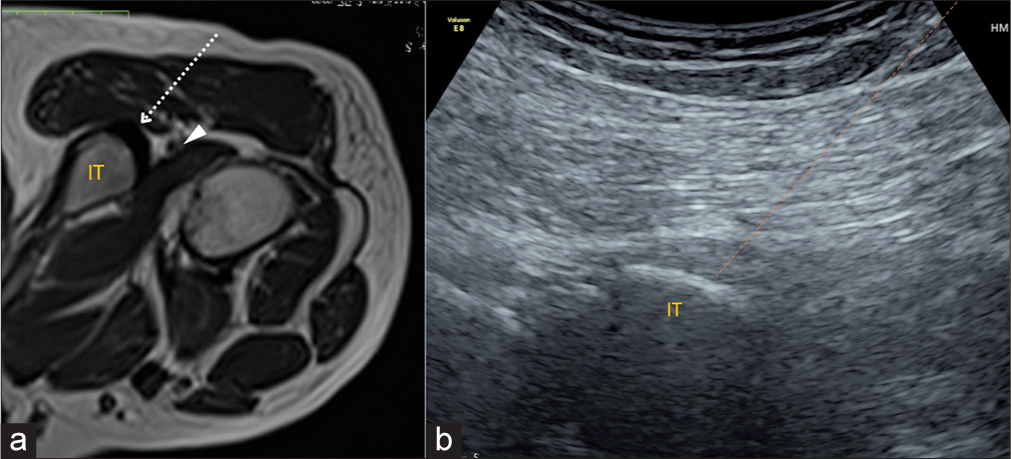
- (a) Platelet-rich plasma injection into the hamstring tendon origin. (a) Axial T1-weighted magnetic resonance image of the hip with the patient in the prone position. Needle trajectory-dotted arrow from the lateral to medial aspect into the hamstring tendon origin. The IT serves as an osseous backstop for the needle. The sciatic nerve (arrowhead) travels in close proximity to the hamstring tendon origin and must be visualized and avoided during the injection procedure. (b) Transverse ultrasound image shows the needle tract (orange dotted line). IT: Ischial tuberosity.
Ischiogluteal bursitis
Ischiogluteal bursitis is characterized by a fluid collection located deep beneath the inferior gluteus maximus muscle. US reveals fluid overlying the ischial tuberosity confirming the diagnosis.[1,11]
Technique: Patient is in prone position. A 22-gauge spinal needle guided in-plane with the transducer from lateral to medial approach. The needle tip is positioned just superficial to the common hamstring tendon origin. The injection is given as a bolus [Figure 12].
Injectate: Total volume of 5 mL–4 mL 2% lidocaine+1 mL of depomedrone.
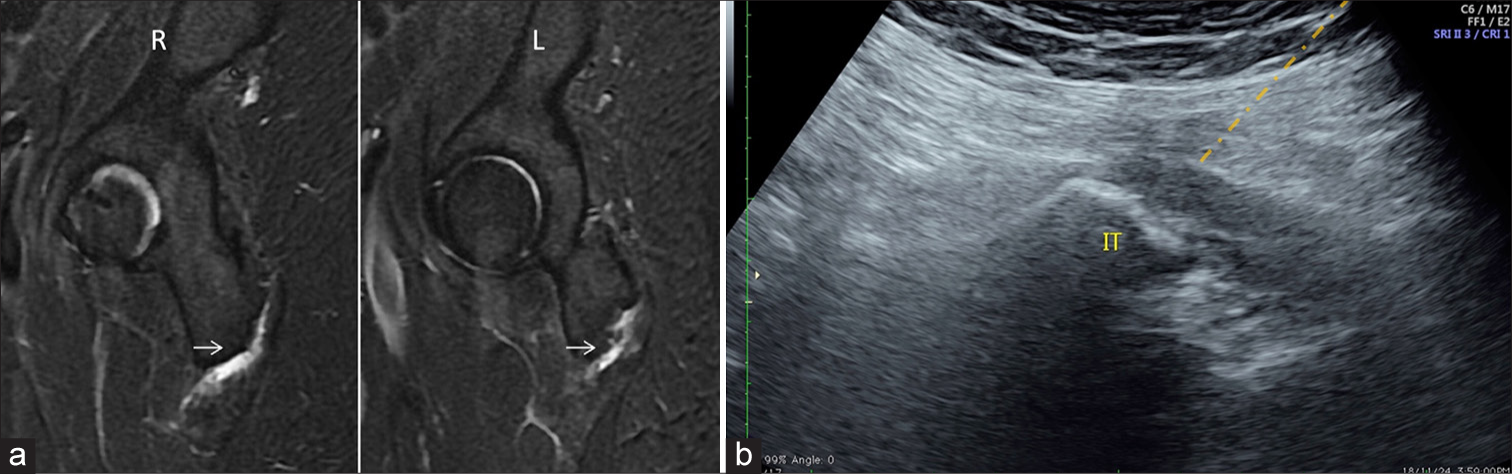
- (a) Sagittal magnetic resonance image of right-R and Left-L hip reveals fluid within the bilateral ischiogluteal bursa suggestive of bursitis. (b) Ultrasound image at the corresponding level shows the orange needle tract for injection. IT: ischial tuberosity. Hamstring origin is outlined in red.
Piriformis muscle
Piriformis syndrome is a debated condition where an abnormal piriformis muscle near the greater sciatic notch compresses the sciatic nerve, causing pain. It may result from muscle hypertrophy, inflammation, or anatomical variations. Conservative treatments include stretching and massage. In refractory cases, US -guided perineural injection and hydrodissection with a local anesthetic, corticosteroids, and saline provide safe, effective symptom relief by reducing nerve compression and inflammation.[1,20]
Technique: The patient is in the prone position. Using a linear or convex probe, sciatic nerve is identified close to the ischial tuberosity where it is situated between the gluteus maximus and pelvitrochanteric muscles, just before reaching the piriformis muscle. Insert a 20–22 G × 9 cm spinal needle using an in-plane approach from the lateral to the medial side.
Injectate: Inject a mixture of 20 mL of corticosteroid, lidocaine, and saline around the nerve to facilitate hydrodissection of the peri-sciatic fascial planes [Figure 13].
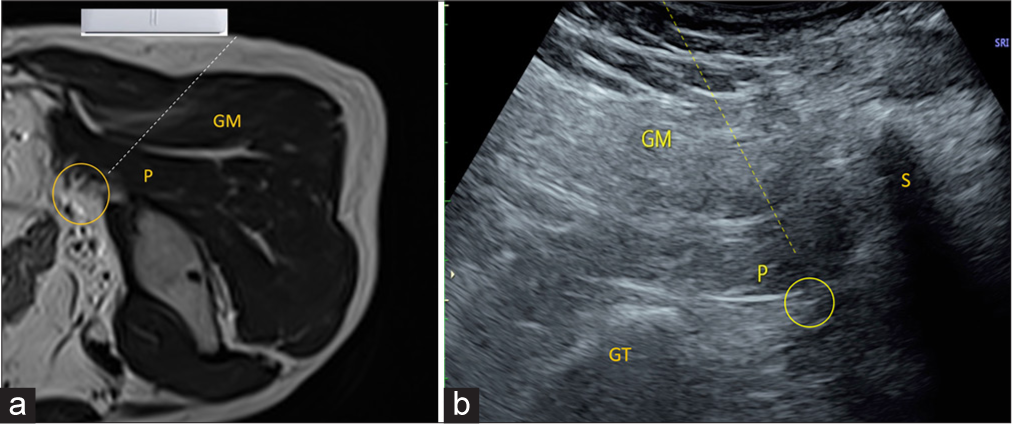
- (a) Axial T2-weighted magnetic resonance image sequence shows probe positioning in prone positioning for piriformis injection. P: Piriformis, GM: Gluteus maximus, Yellow circle demonstrates sciatic nerve. Dotted line demonstrates needle track. (b) Ultrasound image demonstrates needle tract for sciatic nerve hydrodissection. The yellow dotted line demonstrates the needle track. P: Piriformis, GM: Gluteus Maximus, Yellow circle demonstrates sciatic nerve. GT: Greater Trochanter, S: Sacrum.
Ischiofemoral impingement
It is the narrowing of the ischiofemoral and quadratus femoris spaces when the distance between the ischial tuberosity and lesser trochanter is reduced, leading to symptomatic ischiofemoral impingement. This condition presents as muscle edema in acute cases and fatty degeneration in chronic cases.[1,21]
The sciatic nerve lies medial, superficial to the quadratus femoris muscle and lateral to the origin of the hamstring tendons, which should be avoided to prevent accidental injury during needle insertion.
Injection technique: Patient in prone position. The quadratus femoris muscle can be found situated between the ischial tuberosity and the lesser trochanter, deep to the gluteus maximus muscle [Figure 14].
Injectate: Total volume of 5 mL of 2% lidocaine and 1 mL of methyl prednisolone.
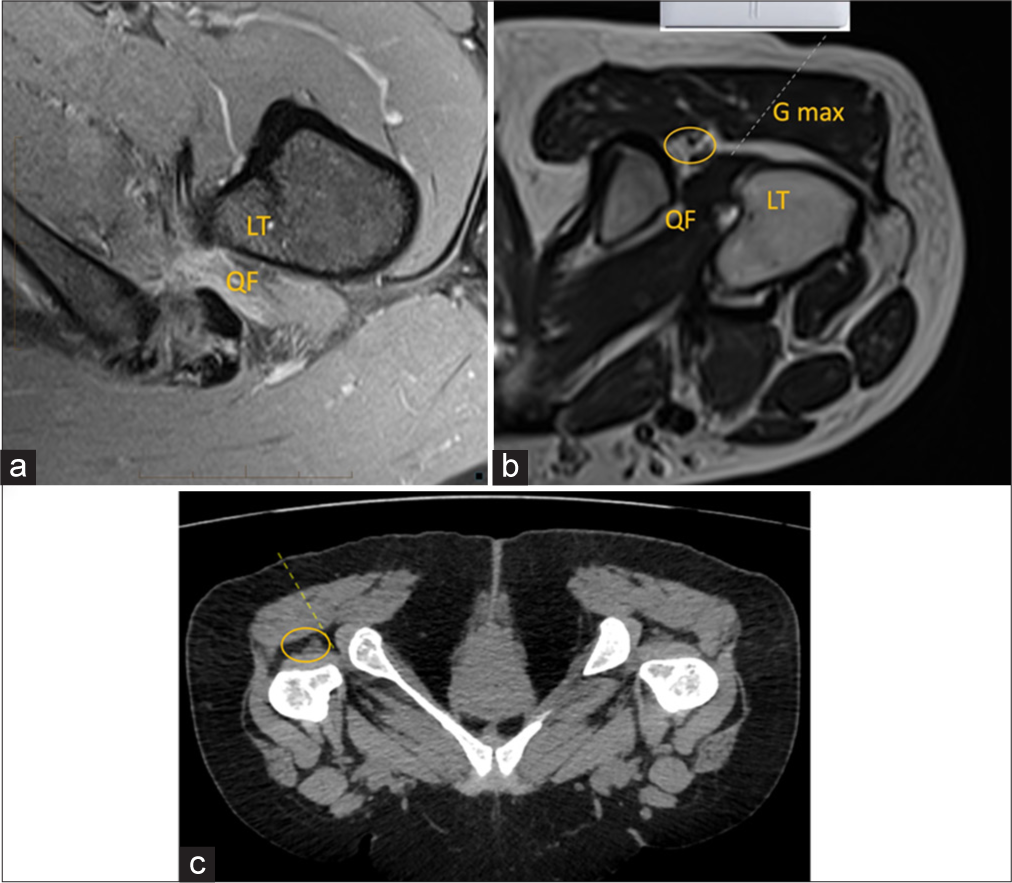
- (a) Axial proton density fat saturated magnetic resonance image (MRI) image depicts edema in the quadratus femoris muscle and narrowing at the ischiofemoral space representing impingement. (b) Axial T2-weighted MRI sequence shows probe positioning in prone position for ischiofemoral space injection. QF: Quadratus femoris, Gmax: Gluteus maximus, LT: Lesser trochanter. Dotted line is the-needle trajectory. Yellow circle demonstrates sciatic nerve. (c) Axial computed tomography reformatted images depicting the needle track. Yellow circle demonstrates sciatic nerve.
CT-guided ganglion impar block
The ganglion impar, a solitary retroperitoneal structure comprising 4–5 small sacral ganglia located at the terminal end of the sympathetic chain, is positioned anteromedial to the sacral foramina. It is continuous with the abdominal sympathetic trunk.[22,23]
The ganglion impar block is indicated for the diagnosis and management of visceral or sympathetic-maintained pain in the perineal and coccygeal regions.[23]
-
Technique:
Patient is instructed to empty the bladder and rectum and placed in prone position with a pillow under the abdomen to allow flexion of the lumbosacral spine
CT is done for pre-injection planning
Needle localizer is placed on the skin to identify sacrococcygeal disc
Needle Insertion: A 25 G, 9 cm long spinal needle is inserted 6–9 cm from the midline
0.5 mL non-ionic contrast medium is required to be injected to confirm placement
Post-injection imaging to confirm needle tip position [Figures 15 and 16].
Injectate: Total volume of 3–5 mL of 2% lidocaine and 1 mL of methyl prednisolone.
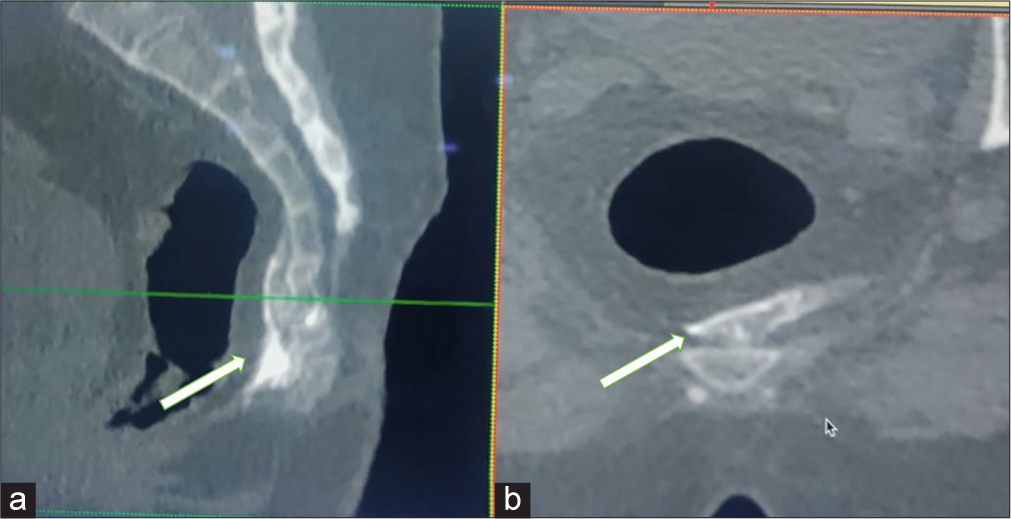
- Sagittal (a) and axial (b) reformatted computed tomography images of the pelvis show the final spread of injectate (arrow) mixed with non-ionic contrast for ganglion impar block in precoccygeal space.
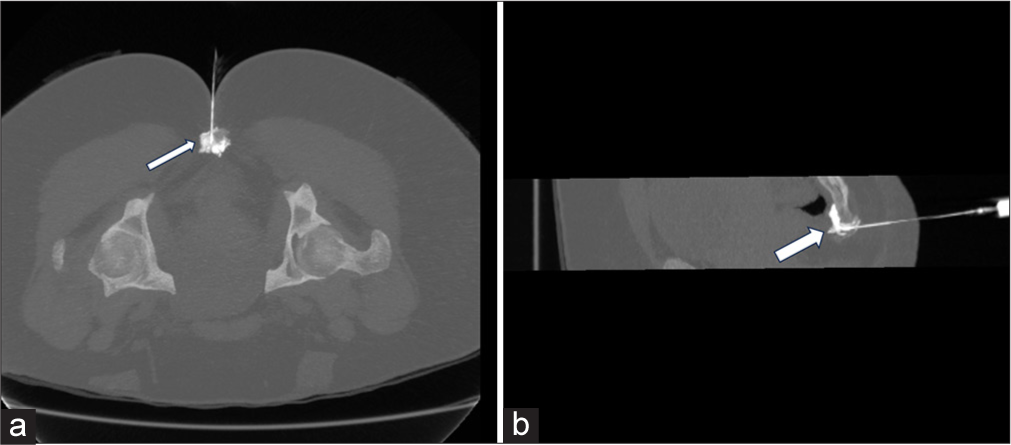
- The patient is placed in a prone position. Axial (a) and sagittal (b) computed tomography in maximum intensity projection depict needle position and spill of nonionic iodinated contrast (arrow) in pre coccygeal plane for ganglion impar block.
CONCLUSION
The complex anatomy of the hip presents challenges in both diagnosis and treatment. Image-guided injections, using US and CT, are essential for precise diagnosis and targeted therapy, including corticosteroid and PRP injections for pain relief and tissue healing. Accurate biopsy and aspiration techniques help identify infections and tumors, ensuring appropriate management.
US -guided nerve blocks provide effective pain control, while PRP promotes tendon healing. Bursal and perineural injections address pain syndromes such as sciatic pain. These customized interventions improve patient outcomes, with safety ensured through meticulous technique and continuous monitoring.
Radiologists play a crucial role in diagnosing and managing hip pain, using US and CT guidance to perform precise percutaneous interventions that enhance treatment efficacy.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- US-guided musculoskeletal interventions in the hip with MRI and US correlation. Radiographics. 2020;40:181-99.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided musculoskeletal interventions for the most common hip and pelvis conditions: A step-by-step approach. Semin Musculoskelet Radiol. 2019;23:e58-67.
- [CrossRef] [Google Scholar]
- Local and systemic side effects of corticosteroid injections for musculoskeletal indications. AJR Am J Roentgenol. 2024;222:e2330458.
- [CrossRef] [PubMed] [Google Scholar]
- Musculoskeletal applications of platelet-rich plasma: Fad or future? AJR Am J Roentgenol. 2011;196:628-36.
- [CrossRef] [PubMed] [Google Scholar]
- The use of ultrasound-guided platelet-rich plasma injections in the treatment of hip osteoarthritis: A systematic review of the literature. J Ultrason. 2018;18:332-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomically based guidelines for core needle biopsy of bone tumors: Implications for limb-sparing surgery. RadioGraphics. 2007;27:189-205. discussion 206
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided biopsy of bone: A radiologist's perspective. AJR Am J Roentgenol. 2008;190:W283-9.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of the hip and bony pelvis: Techniques and applications. Available from: https://link.springer.com/book/10.1007/978-3-031-76546-9 [Last accessed on 2025 Jan 20]
- [Google Scholar]
- Computed tomography versus fluoroscopic guided-sacroiliac joint injection: A prospective comparative study. Insights Imaging. 2021;12:38.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided intra-articular hip injections. 2022. Available from: https://www.nysora.com/pain-management/ultrasound-guided-intra-articular-hip-injections [Last accessed on 2024 Oct 16]
- [Google Scholar]
- Ultrasound-guided musculoskeletal interventional procedures around the hip: A practical guide. J Ultrason. 2023;23:15-22.
- [CrossRef] [PubMed] [Google Scholar]
- Injectable viscoelastic supplements: A review for radiologists. AJR Am J Roentgenol. 2017;209:883-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided blocks of the ilioinguinal and iliohypogastric nerve: Accuracy of a selective new technique confirmed by anatomical dissection. Br J Anaesth. 2006;97:238-43.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided ilioinguinal and iliohypogastric nerve block, a comparison with the conventional technique: An observational study. Saudi J Anaesth. 2015;9:293-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided genitofemoral nerve block for inguinal hernia repair in the male adult: A randomized controlled pilot study. Minerva Anestesiol. 2018;84:189-95.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided iliopsoas bursal injections for management of iliopsoas bursitis after total hip arthroplasty. J Arthroplasty. 2023;38:S426-30.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185:940-3.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided nerve block for pudendal neuralgia: Diagnostic and therapeutic implications. AJR Am J Roentgenol. 2014;203:196-200.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4:17-23.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided piriformis muscle injection for the treatment of piriformis syndrome. Turk Neurosurg. 2014;24:471-7.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided quadratus femoris injection for ischiofemoral impingement. Eur Radiol. 2023;33:3956-60.
- [CrossRef] [PubMed] [Google Scholar]
- Ganglion impar block in patients with chronic coccydynia. Indian J Radiol Imaging. 2017;27:324-8.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided injection for ganglion impar blockade: A radiological approach to the management of coccydynia. Clin Radiol. 2010;65:21-5.
- [CrossRef] [PubMed] [Google Scholar]







