Translate this page into:
Computed tomography guided radiofrequency ablation of osteoid osteoma in technically challenging locations: Tracking through the opposite cortex

*Corresponding author: Dharmendra Kumar Singh, Department of Radiodiagnosis, VMMC and Safdarjung Hospital, South Delhi, New Delhi - 110 029, Delhi, India. dksinghrad@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Krishna LG, Rustagi A, Kumar N, Kumar V, Singh DK. Computed tomography guided radiofrequency ablation of osteoid osteoma in technically challenging locations: Tracking through the opposite cortex. Indian J Musculoskelet Radiol 2021;3(1):34-8.
Abstract
Computed tomography (CT)-guided percutaneous radiofrequency (RF) ablation is an established minimally invasive treatment option of osteoid osteoma. The standard technique involves the percutaneous advancement of an RF probe under CT-guidance through the cortex overlying the nidus of osteoid osteoma in a plane oriented perpendicular to the cortex. In certain specific scenarios, aiming the osteoid osteoma nidus through the overlying cortex is not feasible either due to technical limitations or due to the potential risk of complications. The nidus can be approached through the opposite unaffected cortex in these situations, obviating the need for surgical excision. Performing CT-guided RF ablation of osteoid osteoma through the opposite unaffected cortex is a technically demanding procedure; and, thus requires a high level of expertise. There is a paucity of literature highlighting the RF ablation procedure in technically challenging locations and thus forms the cornerstone of our current technical note.
Keywords
Osteoid osteoma
Radiofrequency ablation
CT-guided radiofrequency ablation of osteoid osteoma
INTRODUCTION
Osteoid osteoma is a benign osteoblastic bone tumor affecting males more than females in the second and third decade of life. More than half of osteoid osteomas are seen in femoral or tibial diaphysis. These painful tumors present with characteristic clinical symptoms of dull aching nocturnal pain which readily responds to aspirin and classical radiological findings of osteoid nidus. For the treatment of osteoid osteoma, percutaneous computed tomography (CT)-guided radiofrequency ablation (RFA) technique is preferred over fluoroscopy-guided RFA or en bloc surgical excision due to its superior nidus visualization, reduced bone loss, and less in-hospital stay.[1-5] The standard CT-guided RFA involves approaching the nidus through the cortex closest to the nidus as described by many investigators.[5-8] Complete ablation of the nidus is the key for successful clinical outcome. Technical success is defined as cannulation of the bone within a 2 mm radius of the nidus. This approach has been widely successful and has rendered CT-guided RFA as a safe, minimally invasive, day care procedure for the treatment of osteoid osteoma.[1-5] One of the major challenges of the standard procedure is to achieve technical success when the lesion is not amenable for such approach as per standard technique.[1,2] These situations primarily arise when either excessive amount of perilesional sclerosis makes approaching the nidus from the overlying cortex technically infeasible or due to the presence of vital structures along the RF probe trajectory. This article purports to illustrate technically difficult scenarios in which CT-guided RFA in intracortical osteoid osteomas of long bones was not possible as per standard technique and the lesions were approached through the opposite unaffected cortex.
TECHNIQUE
While performing CT-guided RFA of osteoid osteoma, the exact location of the nidus, cortical topography, surrounding soft-tissue anatomy, adequate space for operator maneuverability, and anesthetic factors are the key determinants in deciding the best course to approach the nidus. In long bones, the safest method is to approach the lesion from the opposite unaffected cortex whenever the nidus is localized in the posterior and medial cortex of humerus, posterior and medial cortex of femur, and anteromedial cortex of tibia. In the following sections, we present illustrative case examples with discussion of CT-guided RFA of osteoid osteomas in technically challenging location of humerus, femur, and tibia, showcasing lesion approach through the opposite unaffected cortex. CT-guided RFA was performed by a team of interventional radiologist, anaesthesiologist, and specialized radiologic technologist. A written informed consent was obtained from all patients. General anesthesia was administered in all patients. No postoperative complications were observed.
Illustrative case 1
A 19-year-old male with osteoid osteoma in the right proximal humerus underwent CT-guided RFA. The nidus of the tumor was localized on the endosteal surface of posterior cortex with overlying cortical thickening [Figure 1]. A direct approach to the nidus through posterior cortex [Figure 1a dotted red line]” was not recommended as the operator had to drill the full thickness of thickened posterior cortex making the procedure technically arduous and inherently prone to risk of iatrogenic fractures. Furthermore, chances of axillary nerve injury and increased anesthetic risk involved in prone position makes this approach unfavorable. A medial approach “[Figure 1a dotted yellow line]” was not feasible due to high risk of neurovascular bundle injury and less maneuvering space due to hindrance by the chest wall. A lateral approach “[Figure 1a dotted green line]” through middle deltoid muscle is not preferred due to likelihood of axillary nerve injury which normally courses from posterior to anterior aspect. An anterior approach “[Figure 1a dotted blue line]” through anterior deltoid muscle, and lateral to the deltopectoral groove, traversing the unaffected anterior cortex was considered the safest trajectory. CT-guided RFA was successfully performed through anterior approach “[Figure 1b]”.

- (a and b) CT-guided RF ablation in a 19-year-old male with osteoid osteoma in posterior cortex of the right upper humeral diaphysis. (a) Four possible needle trajectory approaches can be defined for the lesion. Posterior (dotted red line), lateral (dotted green line), and medial (dotted yellow line) approaches were not adopted due to possible anesthetic complications, technical difficulty due to excessive sclerosis and risk of neurovascular bundle injury, respectively. Anterior approach (dotted blue line) was selected due to technical feasibility and the lower risk of complications. (b) The insulated needle (white arrow) was introduced with subsequent insertion of RF probe up to the nidus (black arrow).
Illustrative case 2
A 12-year-old male patient with intracortical osteoid osteoma in proximal diaphysis of the left femur underwent CT-guided RFA. The nidus was located on the endosteal surface of the medial femoral cortex with adjoining cortical and medullary sclerosis “[Figure 2]”. A standard approach “[Figure 2a dotted red line]” from the medial aspect was difficult considering close vicinity to femoral neurovascular bundle and less operator maneuverability as pillow wrapped in sterile sheet was kept between the thighs to avoid thermal injury due to skin-skin contact. Posterior approach “[Figure 2a dotted blue line]” was avoided owing to potential risk of sciatic nerve injury. In addition, prone position required in this approach may increase the risks of anesthetic complications. The anterior cortex was thickened due to sclerosis and drilling the anterior thick cortex to reach the nidus would again increase the risk of iatrogenic fracture. The topography of anterior femoral cortex is triangular in shape with steep surface that increases the chances of needle slippage if anterior cortical approach “[Figure 2a dotted green line]” is adopted. Approaching the lesion through vastus lateralis muscle anterior to lateral intermuscular septum, traversing the lateral unaffected cortex “[Figure 2a dotted yellow line]” in lateral decubitus position (to approach the lesion in vertical axis) was deemed most appropriate in this scenario and the procedure was successfully performed through this approach “[Figure 2b]”.
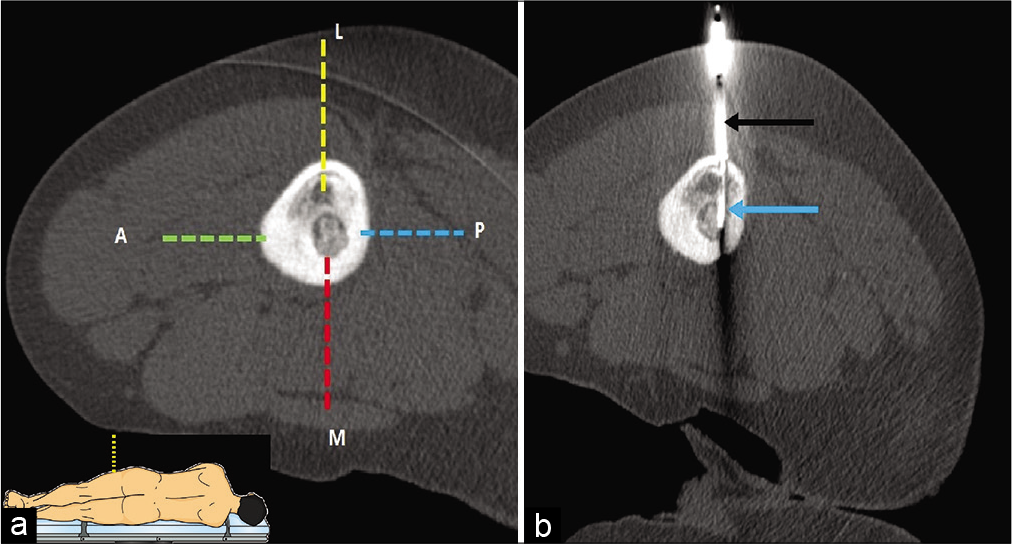
- (a and b) CT-guided RF ablation in a 12-year-old male with osteoid osteoma in medial cortex of the left femoral diaphysis (lateral decubitus position). (a) Four possible needle trajectory approaches can be defined for the lesion. Anterior (dotted green line), medial (dotted red line), and posterior (dotted blue line) approaches were not adopted due to risk of needle slippage, less operating space, and risk of injury to neurovascular bundle respectively. Lateral approach (dotted yellow line) was selected due to technical feasibility and the lower risk of complications. The schematic diagram of lateral decubitus position has been demonstrated in inset. (b) The insulated needle (black arrow) was introduced with subsequent insertion of RF probe up to the nidus (blue arrow).
Illustrative case 3
A 26-year-old male with osteoid osteoma of the left tibial tuberosity was referred for CT-guided RFA. The nidus was located in the medial aspect of tibial tuberosity with surrounding sclerosis. Three possible needle trajectory approaches were defined in this patient. Due to the reduced skin-bone distance, the presence of only thin layer of subcutaneous fat and lack of any muscle compartment, approaching the nidus through the anterior and medial cortex “[Figure 3a dotted green and red lines]” could lead to a high risk of skin burns and subsequent non-healing ulcers and secondary infections jeopardizing the success of the procedure. The third approach “[Figure 3a dotted yellow line]” was therefore preferred in this scenario through lateral cortex. The presence of muscular layer in needle trajectory and reduced perilesional sclerosis on the lateral aspect decreased the chances of fracture and thermal complications. Technical success was subsequently achieved through the desired probe trajectory “[Figure 3b]”.
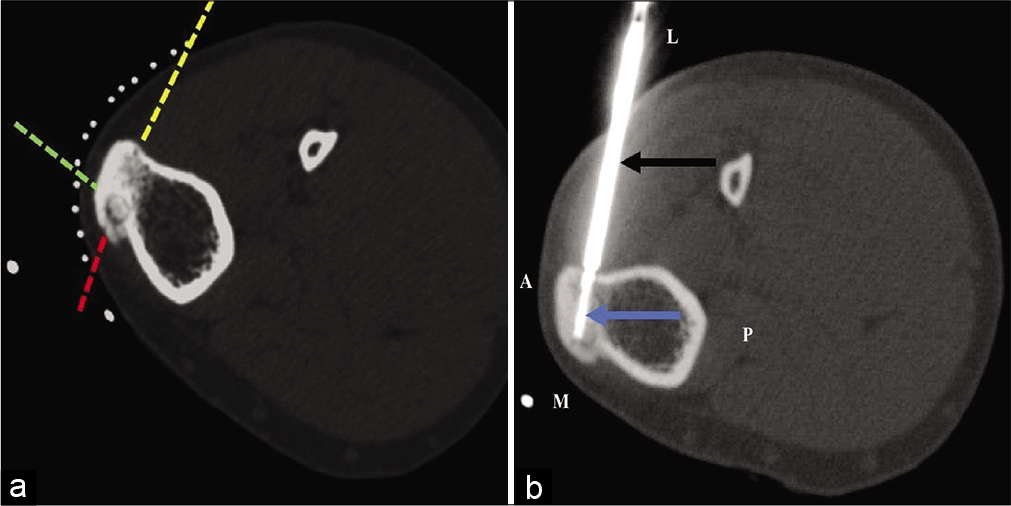
- (a and b) CT-guided RF ablation of osteoid osteoma of the left tibia in a 26-year-old male. (a) The nidus is located in the anteromedial aspect of tibial tuberosity. Three possible needle trajectory approaches can be defined (dotted red, yellow, and green lines) for this lesion. The standard technique involves approaching this lesion from the medial (dotted red line) or anterior tibial cortex (dotted green line), which is not appropriate for this patient. Instead, approaching the lesion from the lateral unaffected opposite tibial cortex (dotted yellow arrow) was preferred. (b) An insulated needle (black arrow) was introduced with subsequent insertion of RF probe (blue arrow) up to the nidus.
DISCUSSION
CT-guided percutaneous RFA is the current standard of care for the treatment of osteoid osteoma. The standard technical procedure of CT guided RFA of extra-articular non-spinal osteoid osteomas involves percutaneous advancement of an insulated needle under CT guidance from the skin to the nidus of the lesion. This is followed by replacing the stylet with an RF probe with subsequent ablation. Careful planning the needle trajectory is an important determinant of technical success. A needle trajectory through the cortex closest to or directly overlying the nidus in a plane oriented perpendicular to the cortex is generally preferred for most lesions.[1-5] However, certain scenarios may preclude this standard approach and invoke the need for trajectory modifications involving insertion of needle through an unaffected cortex, opposite to or farthest from the lesion. These scenarios can either be due to inadequate operating space, nidus location, unfavorable cortical topography, a reduced skin to nidus distance, presence of extensive sclerosis, or due to the presence of vital neurovascular structures in the close vicinity of the lesion.[5-8]
Location of the nidus is a critical factor in determining the needle trajectory.[4] Approaching the lesion from the unaffected opposite cortex is most amenable in cortical variety of osteoid osteoma, where an approach from the overlying cortex is technically infeasible. Intramedullary osteoid osteoma usually harbors a more centrally located nidus and be easily approached by either cortex. On the other hand, approaching a subperiosteal osteoid osteoma from the opposite cortex may result in high chances of iatrogenic fracture as both cortices have to be compromised before the opposite subperiosteal location is reached. The other lesion-related major deterrent in approaching the nidus through the direct overlying cortex is the presence of excessive sclerosis, which is commonly associated with cortical osteoid osteomas. Excessive amount of perilesional sclerosis may pose difficulty in successful nidus cannulation and is frequently associated with hardware related complication such as needle breakage or needle slipping. Another common technical difficulty arises when standard trajectory planning is not possible due to the proximity of vital neurovascular structures near the lesion. It is well established that a nidus located within 10 mm from skin, neurovascular bundle, cartilage should prompt a trajectory modification.[1-5] For instance, if the nidus of the lesion is located on the medial or posteromedial cortex of femur. A direct breech of the medial cortex is not safe due to the presence of neurovascular bundle on the medial aspect of the thigh. A breech of the lateral cortex with subsequent advancement of the insulated needle through the marrow into the medial cortex is the only safe technical approach in this scenario.
Although, insertion of the needle through the unaffected opposite cortex is a viable technical option where a direct approach is not feasible. This approach is technically demanding and has several limitations. One of the major risks associated with this approach is increased rates of iatrogenic fractures.[1,2,6] As a major segment of the bone is breeched during the procedure, chances of significant bone weakening, fractures are substantially high. In our experience, use of bone drill with 2 mm drill bit to advance the needle into the nidus is a better technical option as compared to use of hammer. This is based on the rationale that drill use results in a precise small breech in cortex while using a hammer generate more uncontrolled disruptive forces which may increase fracture rate. Other important consideration while using this approach includes the need for higher accuracy and increase in the number of trajectory modifications. Increase in trajectory modifications may be attributed to two factors. First, the increased distance between the skin and the lesion may require frequent trajectory modifications. Second, the density of the cortical bone is widely different from marrow density. Advancing the needle from a “hard” cortex into a “soft” marrow and back to the cortex may result in changes in needle angulation requiring alterations in the course of needle trajectory. These changes in needle alterations may further reflect as increased number of scans required, higher procedural times, and increased radiation dose. Due to these factors, this approach requires higher expertise and is associated with a relatively long learning curve. Another important factor is the increase likelihood of marrow burns which may occur due to heat conduction from the tip of the RF probe along the length of the needle passing through the bone marrow.[6] Use of completely insulated needle can reduce the chances of “marrow burns.”
CONCLUSION
In conclusion, CT-guided RFA of osteoid osteoma from the unaffected opposite cortex is a viable alternative wherein standard technical approach is not possible. A CT-guided RFA of osteoid osteoma of long bones through “unaffected opposite cortex” may be the safest technical approach when the nidus is located on the medial and posterior cortex of femur and humerus or antero-medial cortex of tibia. Achieving technical success with this approach requires a high level of expertise as multiple trajectory modifications are often required. Use of bone drill instead of hammer has been found to reduce the procedure time as well as incidence of needle fractures and iatrogenic bone fractures in our experience. Defining all the possible needle trajectories during pre-interventional planning and listing potential complications on a case to case basis is imperative to identify the best possible technical approach.
Acknowledgments
The authors would like to thank CT technical team, Mr. Ramesh Chand, Mr. Gurudutt, Mr. Laxman singh Mahar and Mr. Baljeet Singh Yadav for their technical support.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Thermal ablation of osteoid osteoma: Overview and step-by-step guide. Radiographics. 2009;29:2127-41.
- [CrossRef] [PubMed] [Google Scholar]
- Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: Tricks of the trade. AJR Am J Roentgenol. 2002;179:1633-42.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoid osteoma treated with radiofrequency ablation. Adv Orthop. 2015;2015:807274.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided percutaneous radiofrequency ablation of osteoid osteoma: Our experience in 87 patients. Indian J Radiol Imaging. 2017;27:207-15.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided radiofrequency ablation of osteoid osteoma in the long bones of the lower extremity. World J Radiol. 2012;4:278-82.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation of osteoid osteoma: Aiming for excellent outcomes in an Australasian context. J Med Imaging Radiat Oncol. 2018;62:789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation for osteoid osteoma: How we do it. Indian J Radiol Imaging. 2009;19:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography-guided percutaneous radiofrequency ablation of osteoid osteomas: Joining forces of orthopedic oncologists and radiologists. J Tumor Res. 2017;3:113.
- [Google Scholar]






