Translate this page into:
A rare presentation of isolated intraosseous Rosai-Dorfman disease of the tibia – The role of whole-body magnetic resonance imaging in management

*Corresponding author: Ram Sanjith Venkateshwaran, Department of Radiodiagnosis, Ganga Medical Centre and Hospitals Pvt. Ltd., Coimbatore, Tamil Nadu, India. rramsanjith@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Venkateshwaran R, Bhari Thippeswamy P, Jayanthi Kamashi S, Rajasekaran R. A rare presentation of isolated intraosseous Rosai–Dorfman disease of the tibia – The role of whole-body magnetic resonance imaging in management. Indian J Musculoskelet Radiol. 2024;6:140-4. doi: 10.25259/IJMSR_59_2023
Abstract
Rosai–Dorfman disease (RDD) is a rare non-Langerhans histiocytic disorder, usually presenting with enlarged cervical lymph nodes. Extranodal involvement is quite common and synchronously involves the nasal cavity, paranasal sinuses, skin, orbit, central nervous system, and rarely bones. Isolated bone involvement is rare and can present with local symptoms such as pain, swelling, restriction of activity, and occasionally pathological fracture. The most common sites of osseous RDD are the cranium, facial bones, and tibia, in decreasing order of frequency. Imaging findings of primary osseous RDD can be variable and there are no key features to diagnose this entity with certainty. Hence, the primary role of imaging is to plan the management and post-treatment follow-up. Here, we present a case of primary osseous RDD involving tibial diaphysis and emphasize the importance of whole-body magnetic resonance imaging in ruling out systemic involvement.
Keywords
Rosai–Dorfman disease
Whole-body magnetic resonance imaging
Non-Langerhans histiocytic disorder
INTRODUCTION
Rosai–Dorfman disease (RDD) is a non-Langerhans histiocytic disorder that is characterized by the accumulation and overproduction of macrophage-dendritic lineage cells. RDD usually affects lymph nodes and presents with painless cervical lymphadenopathy. Apart from nodal involvement, there can be synchronous extranodal involvement of other structures such as the nasal cavity, paranasal sinuses, skin, orbit, central nervous system, and bones. Primary osseous involvement without lymph node involvement is rare and presents with pain, swelling, and pathological fracture. Radiological findings are typically osteolytic, mixed osteolytic/sclerotic, and intramedullary with surrounding sclerosis. However, the diagnosis is primarily histopathological. We report a case of primary osseous RDD involving tibial diaphysis and emphasize the importance of whole-body magnetic resonance imaging (WB-MRI) in ruling out systemic involvement for optimal management.
CASE REPORT
A 24-year-old female patient presented with complaints of gradually progressive, dull aching and pain in the left leg for 2 months, which aggravated on walking. Local examination revealed tenderness over the distal leg with no local rise in temperature. A systemic examination was non-contributory. A hematological workup revealed raised C-reactive protein and elevated erythrocyte sedimentation rate (ESR).
The plain radiograph and computed tomography (CT) of the left lower leg showed an intramedullary osteolytic lesion with a narrow transition zone in the mid-diaphysis of the left tibia. The lesion was seen to cause endosteal scalloping and cortical tunneling, periosteal reaction. No cortical breach, periosteal reaction, or soft-tissue component was present [Figure 1].
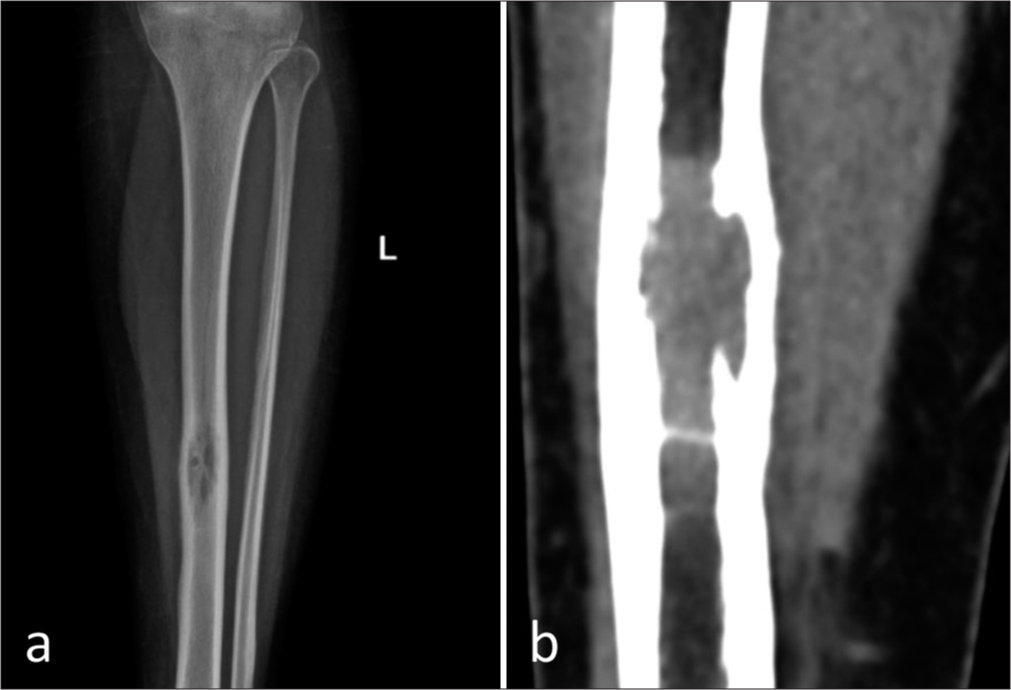
- (a) Plain anteroposterior and (b) computed tomography of the left leg showing a solitary, expansile, intramedullary osteolytic lesion with a narrow zone of transition in the mid diaphysis of the left tibia. The lesion is causing endosteal scalloping and involves anterior and posterior cortices. No obvious periosteal reaction. No cortical break. No soft-tissue component.
The magnetic resonance imaging (MRI) of the left leg depicted a well-defined intramedullary marrow infiltrating lesion in the mid-diaphysis of the tibia, which was hypointense on the T1-weighted image and hyperintense on T2-weighted images with restricted diffusivity. There was no adjacent soft-tissue component [Figures 2 and 3].
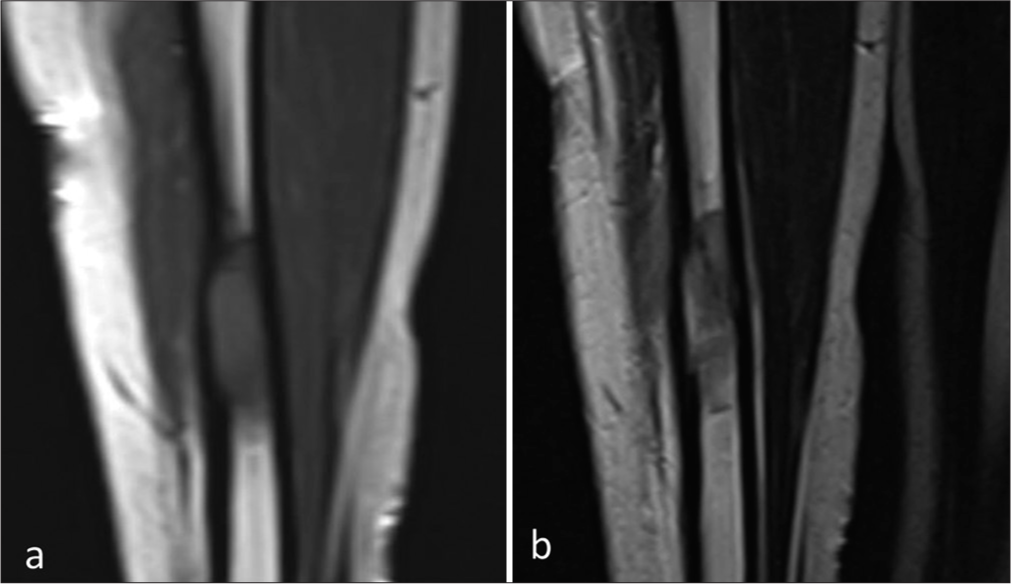
- (a) Coronal T1- and (b) T2-weighted magnetic resonance image showing a well-defined intramedullary marrow infiltrating lesion which is hypointense on the T1-weighted image and hyperintense on T2-weighted image in the mid-diaphysis of the tibia. No adjacent soft-tissue component.
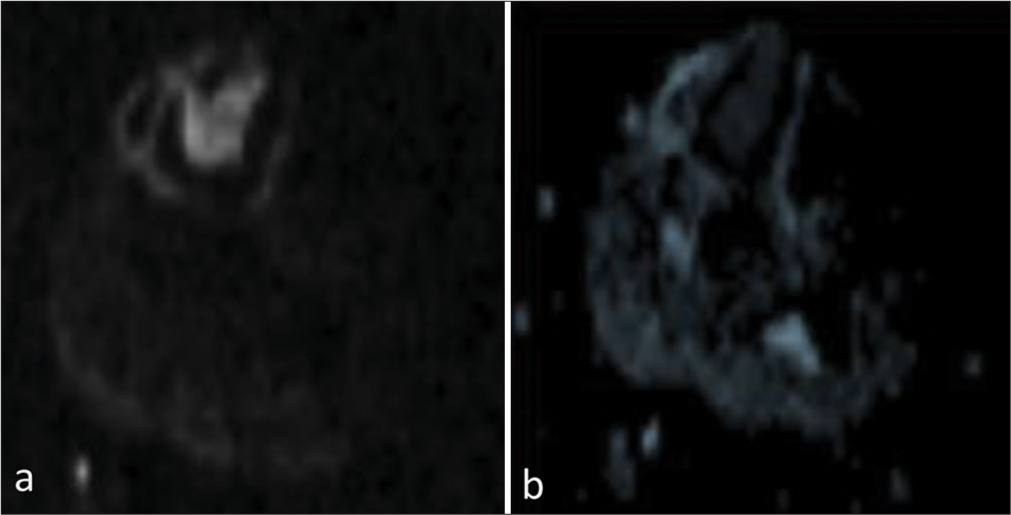
- (a) Diffusion-weighted image and corresponding (b) Apparent diffusion coefficient image showing restricted diffusivity.
Differential diagnoses of Langerhans histiocytosis, lymphoma, and Ewing’s sarcoma were considered.
Histopathological examination of the curetted tissue revealed a heterogeneous infiltrate of histiocytes, plasma cells, lymphocytes, and neutrophils. There were few histiocytes with features suspicious of emperipolesis. No granulomas or any other atypical cells were seen. The histiocytes exhibit positivity for CD163 and patchy positivity for S100. CD1a was negative.
Histopathological examination features were indicative of RDD [Figure 4].
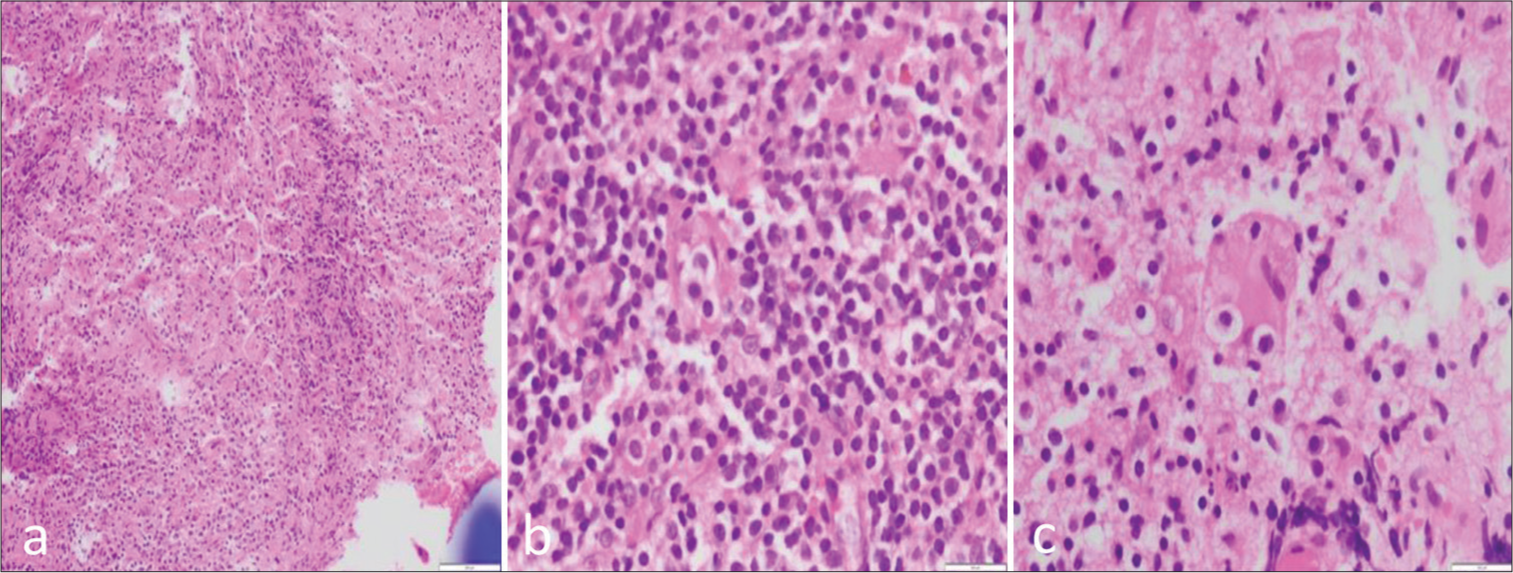
- (a-c) Histopathological sections show fragments of fibro collagenous tissue displaying a heterogeneous infiltrate composed of sheets of histiocytes admixed with plenty of plasma cells, lymphocytes, and neutrophilic microabscesses. The histiocytes are large with pale, foamy cytoplasm, and some of them contain engulfed neutrophils, lymphocytes, and plasma cells suggestive of emperipolesis.
Considering the diagnosis of RDD, the patient was subjected to a WB-MRI. Since the osseous lesion was hyperintense on short-tau inversion recovery (STIR), we used a coronal STIR sequence from head to mid-thigh to look for synchronous lesions elsewhere in the body. There was no synchronous involvement of other systems [Figure 5].
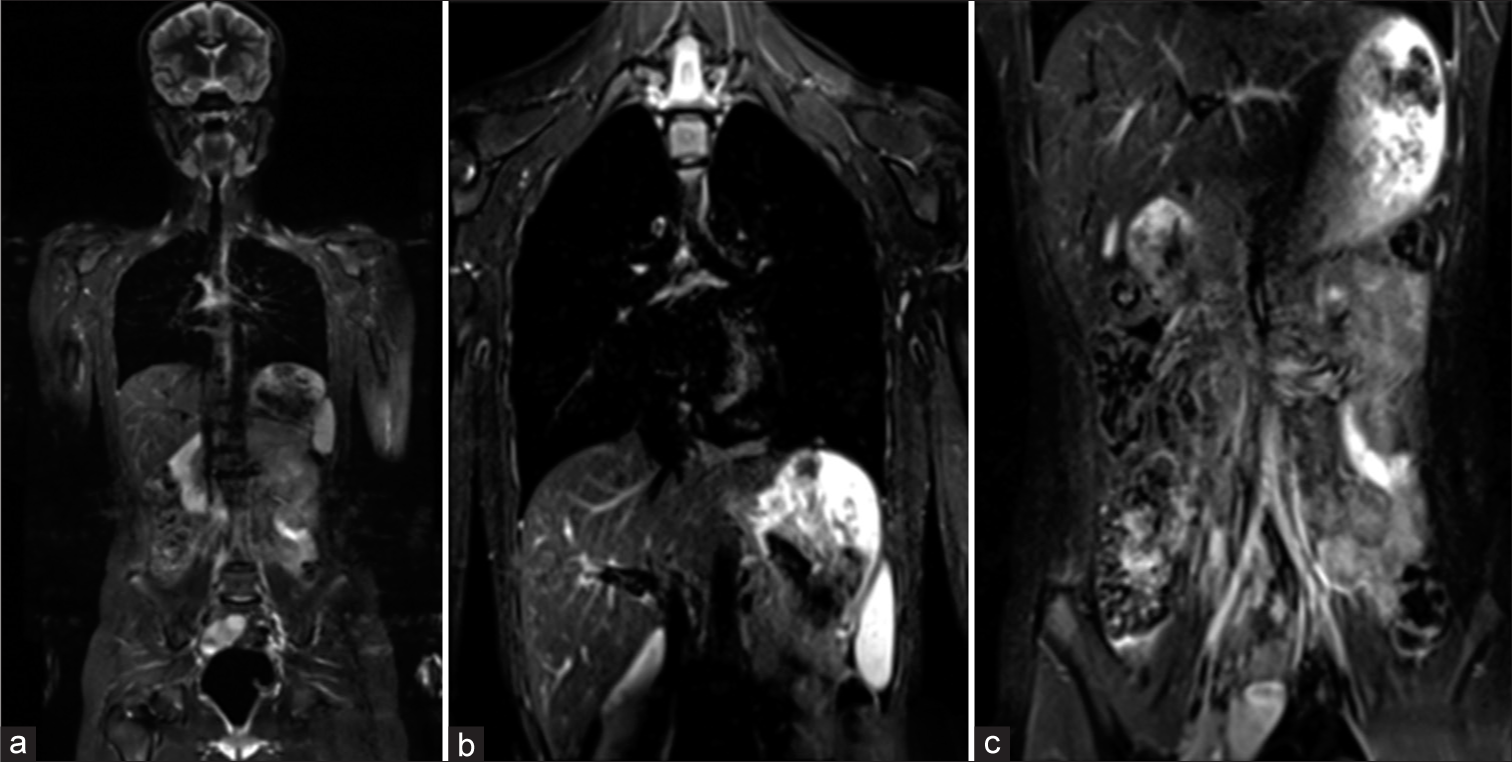
- (a-c) Whole-body magnetic resonance imaging showing no synchronous involvement of the other systems.
In the absence of synchronous involvement by other systems, the patient was planned for curettage of the lesion and bone grafting with dynamic compression plating of the tibia. The patient was followed up for 3 months, and there was no radiological evidence of local or systemic recurrence. The patient is symptom-free and showed allograft-induced osteogenesis [Figure 6].
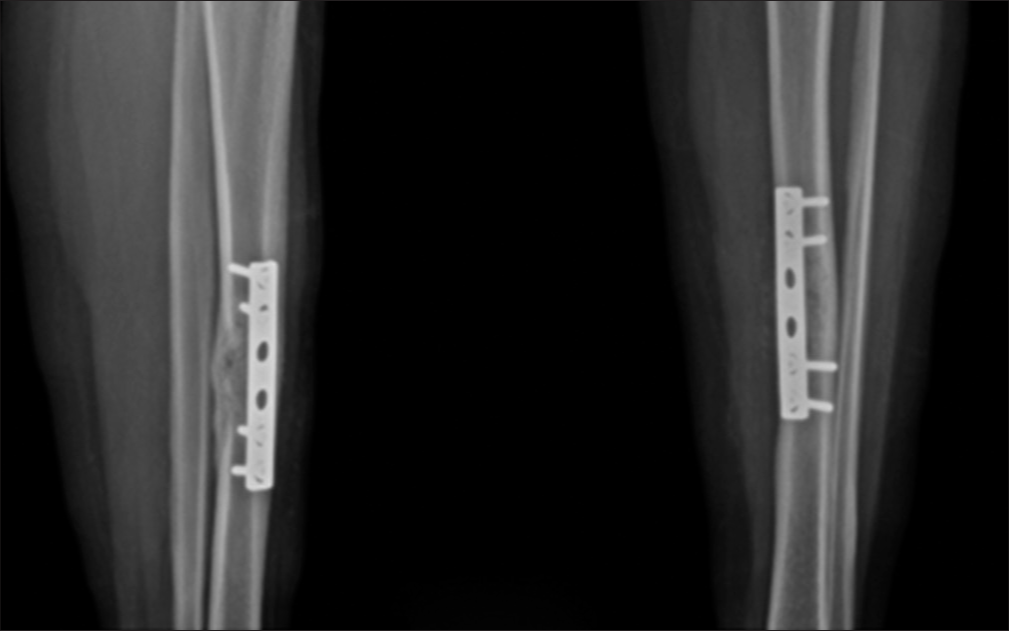
- Post-operative radiograph showing cortical thickening with good osseous consolidation. Plate and screws fixation is in situ.
DISCUSSION
RDD is a rare, non-neoplastic, and self-limiting disease with a poorly understood etiology, predominantly affecting the younger population with a male preponderance. RDD is a nonLangerhans cell histiocyte disorder with sinus histiocytosis and massive cervical lymphadenopathy. An exaggerated immune response to an infective cause or viruses such as parvo B19, human herpes virus, and Ebstein–Barr virus can lead to the proliferation of histiocytes, which is postulated as a probable cause. RDD usually presents with painless cervical lymphadenopathy, with 43% of those cases showing synchronous extranodal involvement. The sites of extranodal involvement are the nasal cavity, paranasal sinuses, central nervous system, skin, and bones. Extranodal manifestations in the absence of nodal involvement are extremely rare.[1]
Isolated osseous RDD mostly occurs in adulthood and is solitary, affecting the skull, tibia, clavicle, sacrum, femur, and bones of the hands and feet. A systematic literature review done by Mosheimer et al. showed 108 patients with osseous RDD, of which 26% had isolated osseous involvement.[2] The most commonly affected bones were the cranium, facial bones, and tibia, in decreasing order.[2]
Isolated osseous RDD presents with pain and swelling. The average interval between the onset of signs and symptoms and the diagnosis of the disease is approximately 3–6 months.[3,4] Hematology may show elevated ESR, white cell count, and hypergammaglobulinemia.[1]
Imaging features of isolated bone RDD are non-specific. On radiographs, osseous RDD is an intramedullary lesion affecting either metaphysis or diaphysis. Lesions are lytic or mixed lytic and sclerotic, which are poorly or sharply marginated. A pathological fracture is usually rare. MRI features include T1 hypointensity, T2 hyperintensity, and restricted diffusivity, indicating high cellularity. The imaging differential diagnosis for a solitary osteolytic or a mixed lytic and sclerotic bony lesion includes metastasis, myeloma, Langerhans histiocytosis, lymphoma, or chronic osteomyelitis.[4]
Since imaging features are non-specific, diagnosis of RDD is mainly by histopathological analysis. A key feature is the process called emperipolesis, where the histiocyte phagocytizes intact lymphocytes and plasma cells. Emperipolesis is a characteristic but not a pathognomonic feature of the disease. Immunohistological markers such as s-100 CD68 positivity and CD1a negativity help in confirming the diagnosis of RDD.[1,4]
The main role of imaging in isolated osseous RDD is to rule out nodal and other extranodal diseases and post-treatment follow-up.[5,6] Patients with isolated bone lesions can be treated with bone curettage and grafting, avoiding systemic medications.[7]
Lu et al., in their retrospective literature review, identified 28 patients who underwent fluorodeoxyglucose FDG positron emission tomography CT scan for systemic assessment and follow-up of RDD.[7] There is no literature highlighting one definitive modality that will rule out the systemic involvement in cases of isolated osseous RDD.
There is a trend of utilizing WB-MRI in cases of metastasis, multiple myeloma, lymphoma, and other hematological disorders both for analyzing pre-treatment disease burden and post-treatment response. Apart from these oncological applications, WB-MRI can be preferred in detecting early changes in systemic conditions like rheumatoid arthritis. Synovitis, bone marrow edema, osteitis, tendinitis, and ligamentous pathologies can be demonstrated early with WB-MRI. The major advantages of WB-MRI include negating the use of radiation and injection of a radioactive tracer. Imaging protocols generally include T1 to look for bone marrow involvement, T2, and STIR for the evaluation of disease in organs other than bone. Diffusion-weighted imaging can be added, which has a high propensity to detect lesions with high cellularity. The major technical issue faced by WB-MRI is related to the field inhomogeneity due to large anatomical coverage in a single scanning station leading to artifacts, which can be addressed by advanced shimming techniques. Realistic issues include being in a closed space and noisy environment for a long time. This can be addressed by reassuring the patient and explaining about this effective imaging technique and seeing it favorably in comparison to other imaging procedures. Our patient was a young female, and the decision to limit the radiation outweighed the above list of disadvantages and prompted us to use MRI for management planning.[8]
We utilized whole-body using STIR sequence to confirm the isolated nature of the disease. Since isolated RDD was confirmed by WB-MRI, curettage, and grafting were sufficient in this young female without any need for oral corticosteroids and immunosuppressants.
CONCLUSION
Primary osseous RDD is a rare entity and may present with non-specific aggressive features in imaging. Although the diagnosis of RDD is mainly based on histopathology, imaging plays a crucial role in management and follow-up. Isolated osseous RDD can be treated with local curettage and bone grafting without any systemic medications. WB-MRI may be useful to rule out synchronous RDD to offer optimal management.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- RosaiDorfman disease: A report of eight cases in a tertiary care center and a review of the literature. Braz J Med Biol Res. 2015;48:6-12.
- [CrossRef] [PubMed] [Google Scholar]
- Bone involvement in Rosai-Dorfman disease (RDD): A case report and systematic literature review. Curr Rheumatol Rep. 2017;19:29.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue Rosai-Dorfman disease: 29 new lesions in 18 patients, with detection of polyomavirus antigen in 3 abdominal cases. Ann Diagn Pathol. 2010;14:309-16.
- [CrossRef] [PubMed] [Google Scholar]
- Extranodal RosaiDorfman disease: Clinicopathologic series of 10 patients with radiologic correlation and review of the literature. Am J Clin Pathol. 2016;145:211-21.
- [CrossRef] [PubMed] [Google Scholar]
- Whole-body diffusion-weighted MRI in a case of Rosai-Dorfman disease with exclusive multifocal skeletal involvement. Skeletal Radiol. 2012;41:709-13.
- [CrossRef] [PubMed] [Google Scholar]
- Rosai-Dorfman disease: Tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-7.
- [CrossRef] [PubMed] [Google Scholar]
- The value of 18F-FDG PET/CT in the systemic evaluation of patients with Rosai-Dorfman disease: A retrospective study and literature review. Orphanet J Rare Dis. 2023;18:116.
- [CrossRef] [PubMed] [Google Scholar]
- Whole-body magnetic resonance imaging: Technique, guidelines, and key applications. Ecancermedicalscience. 2021;15:1164.
- [CrossRef] [PubMed] [Google Scholar]







