Translate this page into:
Case report: Brown tumor, a masquerader of malignancy

-
Received: ,
Accepted: ,
How to cite this article: Hiremath R, Moudgalya A. Case report: Brown tumor, a masquerader of malignancy. Indian J Musculoskelet Radiol 2023;5:56-60.
Abstract
There are various causes of multiple lytic lesions in bone in adults, among which metastases and multiple myeloma top the list. Brown tumor is a rare cause of multiple lytic lesions in bone in the present era. This is due to the early diagnosis of hyperparathyroidism by serological investigations and effective treatment. Brown tumor is named after its gross pathological appearance and is focal reactive osteolysis secondary to elevated parathyroid hormone. We herein present a case of a 36-year-old female with multiple osteolytic lesions with high suspicion of metastases, but a methodical radiological investigation suggested a case of primary hyperparathyroidism with multiple brown tumors. Therefore, brown tumor should always be kept as a differential diagnosis in the case of multiple lytic lesions in bone in adult patients.
Keywords
Brown tumor
Hyperparathyroidism
Chronic renal failure
Osteolytic lesions
Parathyroid adenoma
INTRODUCTION
There are various causes of multiple lytic lesions in bone in adults, among which metastases and multiple myeloma top the list. Brown tumor is a rare cause of multiple lytic lesions in bone in the present era. This is due to the early diagnosis of hyperparathyroidism by serological investigations and effective treatment. Brown tumor is named after its gross pathological appearance and is focal reactive osteolysis secondary to elevated parathyroid hormone. We herein present a case of a 36-year-old female with multiple osteolytic lesions with high suspicion of metastases, but a methodical radiological investigation suggested a case of primary hyperparathyroidism with multiple brown tumors. Therefore, brown tumor should always be kept as a differential diagnosis in the case of multiple lytic lesions in bone in adult patients.
MATERIALS AND METHODS
A 36-year-old female was referred to the Department of Radiodiagnosis for an ultrasound (USG) abdomen and pelvis with a history of the right flank pain lasting for more than 3 months. USG revealed bilateral renal calculi with moderate right hydroureteronephrosis secondary to the upper ureteric calculi. As a routine workup by the urology department, the patient was asked for a chest radiograph posteroanterior (PA) view and spiral computed tomography (CT) kidney ureter bladder (KUB). Chest radiograph revealed right paratracheal stripe widening with homogenous opaque middle mediastinal mass with cervical extension and depicting cervicothoracic sign [Figure 1]. No calcification or other differential densities were noted within the mass. The mass was causing smooth indentation and deviation of the trachea to the left. Well-defined pleural-based nodules were noted along the right 5th, 6th, and 7th ribs indenting the adjacent lung parenchyma [Figure 1]. The rest of the lungs were clear. Diffuse osteopenia was also noted with significant focal osteopenia of lateral ends of clavicles [Figure 1]. With the above radiographic findings, the possibility of mediastinal mass with secondaries in the pleura was considered. However, in view of osteopenia and lateral clavicular bone density loss, parathyroid neoplasm with hyperparathyroidism was also kept a differential.
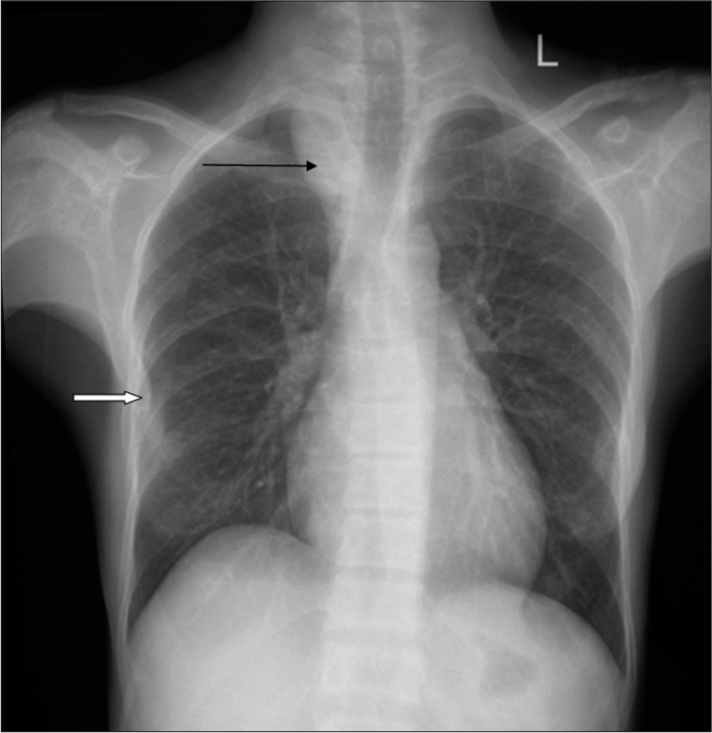
- Antero-posterior chest radiograph showing right paratracheal stripe widening (Black arrow) with homogenous opaque middle mediastinal mass and cervical extension depicting cervicothoracic sign. The mass is causing smooth indentation and deviation of trachea to the left. Well-defined pleural based nodules along the right 5th, 6th, and 7th ribs indenting the adjacent lung parenchyma. (White arrow) Diffuse osteopenia of the bones with focal significant osteopenia of lateral ends of clavicles.
On spiral CT KUB, sonological findings of bilateral renal calculi and right hydroureteronephrosis secondary to multiple upper ureteric calculi were confirmed [Figure 2]. The bone window revealed extensive osteoporosis of the spine, pelvis and proximal femori with multiple well-defined, and variable-sized osteolytic lesions with or without a thin sclerotic rim. The osteolytic lesions showed mixed density within [Figure 3]. No evidence of fractures or soft-tissue extensions was noted.

- Coronal computerized tomography scan of the kidney ureter bladder region depicting left renal calculi (black arrow) and right hydroureteronephrosis (white arrow) secondary to multiple right upper ureteric calculi (black curved arrow).

- Coronal CT scans in bone window at different levels showing extensive osteoporosis of spine, pelvis, and proximal femori with multiple well-defined, variable sized osteolytic lesions with or without thin sclerotic rim. The osteolytic lesions show mixed density within.
For further characterization of the neck mass, CT neck and chest with and without contrast were performed, which revealed a well-defined heterogeneously enhancing right paratracheal soft-tissue density mass lesion immediately caudal to the right lobe of the thyroid gland with mediastinal extension causing tracheal indentation. No obvious evidence of infiltration of mass into the adjacent structures. Multiple well-defined heterogeneously enhancing expansile osteolytic lesions were noted in the mandible, maxilla, and visualized ribs. Mandibular lesions were expansile and showed cortical break with the soft-tissue extension of enhancing tumor mass. Significant alveolar bone destruction around the roots of the tooth resulted in a “floating tooth sign” [Figure 4].

- Axial (a) and coronal (b) contrast-enhanced computerized tomography chest and neck showing well-defined heterogeneously enhancing right paratracheal solid mass lesion (Thick white arrow). (c and d) Expansile mandibular lesion showing cortical break with soft-tissue extension of enhancing tumor mass (curved black arrow). Significant alveolar bone destruction is noted around the roots of tooth resulting in floating tooth sign (straight black arrow).
As part of the skeletal survey, lateral skull and both hands PA view radiographs were also obtained. The skull radiograph lateral view revealed multiple tiny osteolytic lesions with poor definition of tables giving the classical appearance of a “pepper pot skull” [Figure 5]. Hand radiograph revealed classical subperiosteal bone resorption affecting the radial aspects of the middle phalanges of the 2nd, 3rd, and 4th digits bilaterally [Figure 6]. Well-defined osteolytic lesions with thin sclerotic rim were noted in the right 2nd metacarpal, left 5th metacarpal, and the terminal phalanx of the left 1st digit.

- Skull radiograph lateral view showing multiple tiny osteolytic lesions with poor definition of tables of skull giving classical appearance of pepper pot skull.
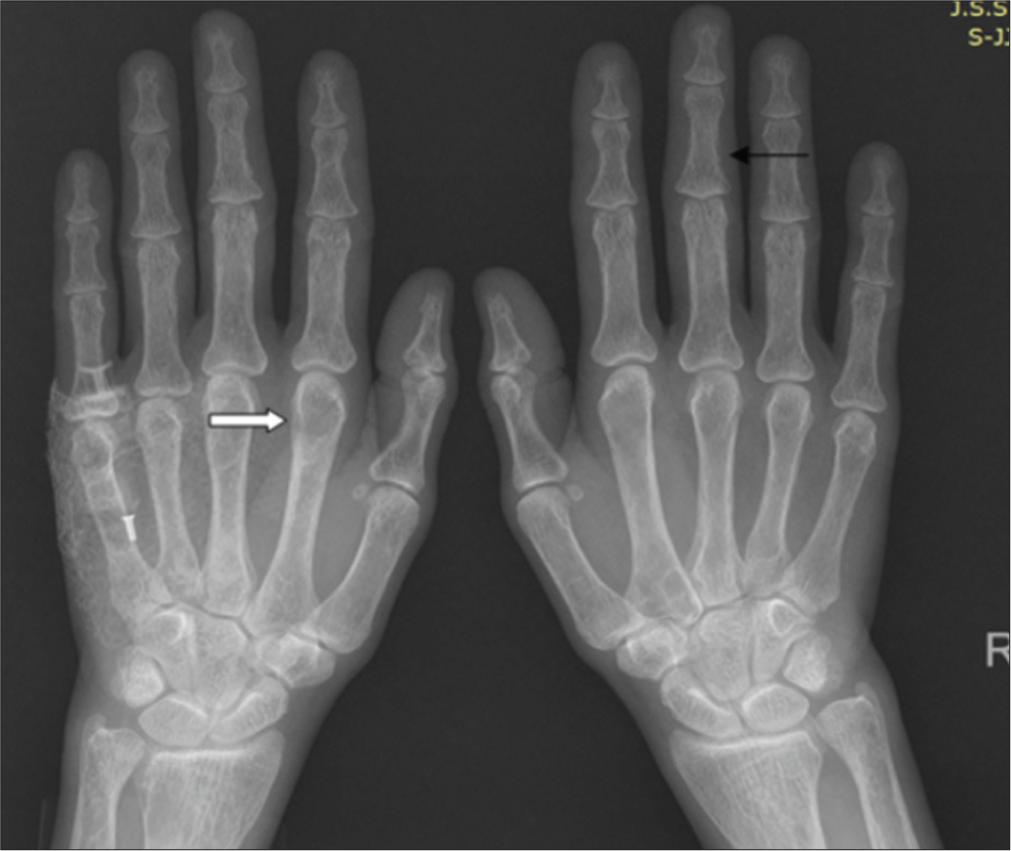
- Hand radiograph reveals subperiosteal bone resorption affecting the ulnar aspect of middle phalanges of 2nd, 3rd, and 4th digits bilaterally (black thin arrow); bones show coarsened trabeculae with well-defined osteolytic lesions with thin sclerotic rim in the right 2nd metacarpal, left 5th metacarpal, and left 2nd metacarpal head (thick white arrow).
On the basis of radiological findings, a diagnosis of primary hyperparathyroidism secondary to the parathyroid neoplasm was made. Radiological findings were supplemented by serological investigations, which revealed serum calcium −11.4 mg/dL, alkaline phosphatase− 3135 U/L, and vitamin D –28.2 ng/dL.
The patient was subjected to the fine-needle aspiration cytology (FNAC) of the mandibular lytic lesion with soft-tissue extension which revealed a giant cell lesion. FNAC of the neck was performed under the USG guidance which confirmed the diagnosis of parathyroid adenoma.
Thereafter, surgical resection of the neck and mediastinal mass was performed. To avoid complications of hungry bone syndrome, the patient was put on a high dose of calcium. Histopathology of the resected mass confirmed the diagnosis of parathyroid adenoma.
DISCUSSION
Osteitis fibrosa cystica or Von Recklinghausen’s disease of bone are other synonyms of a brown tumor. It is a manifestation of primary, secondary or tertiary hyperparathyroidism. Initially, it was assumed that brown tumor is pathognomonic of primary hyperparathyroidism and is seen mainly in this condition. However, later, it was realized that brown tumors can occur in any form of hyperparathyroidism and is more common in secondary hyperparathyroidism due to chronic kidney diseases.[1]
Solitary parathyroid adenoma is the most common cause of primary hyperparathyroidism accounting for approximately 80% of cases, whereas multiple adenoma, parathyroid dysplasia, and parathyroid carcinomas accounts for the rest of the small proportions of cases.[2] The incidence of brown tumor in primary hyperparathyroidism is 3%, whereas, in secondary hyperparathyroidism, it is approximately 2%.[1] Chronic renal failure and vitamin D deficiency cause reduced serum calcium and elevated parathyroid hormone resulting in secondary hyperparathyroidism. Due to early detection and effective treatment of hyperparathyroidism by routine blood investigations, brown tumor has become a rare entity.
Presentation of brown tumor can be as solitary lytic or multiple lytic bone lesions and is considered a sign of poorly controlled disease.[3] A brown tumor may occur in any bone but most commonly affects long bones, facial bones, mandible, pelvis, and ribs.[4]
On gross pathological examination, the lesion appears brown in color secondary to its vascularity, hemorrhage, and deposits of hemosiderin, hence the name. Brown tumor is indistinguishable from giant cell tumors of the bone on histology and is characterized by hypervascular fibroblastic stroma, foci of hemorrhage, and accumulation of osteoclastic giant cells.[2,5]
Radiographically, the brown tumor appears as well-defined solitary or multiple lytic expansile lesions with or without sclerotic borders. These are medullary lesions, hence are located centrally. Computed tomography defines lesions better, especially in complex bones like the pelvis and spine, and also provides more information on the adjacent soft-tissue extension. There are many reviews and case reports of magnetic resonance imaging (MRI) features of brown tumor in the literature. On MRI, brown tumors may appear entirely solid or mixed solid and cystic mass or complex mass with fluid-fluid levels. Solid appearing mass lesions are iso to hypointense on T1- and T2-weighted images. Tumors with fluid-fluid levels are secondary to repeated hemorrhages and hemosiderin deposits. On contrast administration, solid component and septae show enhancement.[6]
Progressive osteoclastic activity in these lesions may lead to cortical destruction resulting in microfractures. Further, progression may lead to pathological fractures with extraskeletal soft-tissue components, mimicking metastatic disease.[4,7]
The treatment of brown tumors is mainly to reduce parathyroid hormone levels. In the case of primary hyperparathyroidism, resection of the tumor will help to heal the brown tumors. The healing process is by osteoblastic activity and remineralization of brown tumors resulting in multiple sclerotic lesions in the healed bone. In post-surgical resection of parathyroid adenoma, patients may land up with hungry bone syndrome secondary to rapid, profound hypocalcemia (<2.1 mmol/L) lasting for more than 4 days. The entity is more common in patients with multiple brown tumors and is treated by administration of a high dose of calcium for up to 1 year.[8]
The gold standard for the diagnosis of brown tumor is biopsy and histopathological confirmation.[1] However, classical radiological features in the presence of elevated parathyroid hormone will suffice the diagnosis. Other features of hyperparathyroidism are subperiosteal bone resorption, subchondral bone resorption, acro-osteolysis, osteopenia, and chondrocalcinosis. In addition to the above findings, soft tissue calcifications and renal calculi might be present in patients with secondary hyperparathyroidism. Histopathology confirmed the diagnosis of parathyroid adenoma.
CONCLUSION
Metastasis and myeloma are always the first differential diagnosis for multiple osteolytic lesions in aged patients. Brown tumor should be kept as a differential diagnosis which may mimic metastases by showing multiplicity, pathological fractures, and soft-tissue extension. Even though biopsy is the gold standard for the diagnosis of brown tumor, an amalgamation of serological and radiological findings may pin the diagnosis.[4,7]
In the current case scenario of a middle-aged female with extensive osteolytic lesions and mediastinal mass, there was a diagnostic challenge with the first differential diagnosis as a malignant neoplasm with lytic bone metastases. To arrive at the appropriate diagnosis, orderly radiological investigations and careful interpretation of the findings with serological correlation are a necessity.
Declaration of patient consent
Patient's consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Osteitis fibrosa cystica mistaken for malignant disease. Clin Exp Otorhinolaryngol. 2013;6:110-3.
- [CrossRef] [PubMed] [Google Scholar]
- Osteolytic lesions (brown tumors) of primary hyperparathyroidism misdiagnosed as multifocal giant cell tumor of the distal ulna and radius: A case report. J Med Case Rep. 2018;12:176.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple 'brown tumors' masquerading as metastatic bone disease. Cureus. 2015;7:e431.
- [CrossRef] [Google Scholar]
- Craniofacial brown tumor as a result of secondary hyperparathyroidism in chronic renal disease patient: A rare entity. J Oral Maxillofac Pathol. 2014;18:267-70.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of brown tumours: A pictorial review. Insights Imaging. 2019;10:75.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hyperparathyroidism having multiple Brown tumors mimicking malignancy. Indian J Endocrinol Metab. 2012;16:1040-2.
- [CrossRef] [PubMed] [Google Scholar]






