Translate this page into:
Diffusion tensor imaging in idiopathic inflammatory myopathies: A case–control study

*Corresponding author: Sonal Saran, Diagnostic and Interventional Radiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. sonalsaranmalik@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saran S, Nandolia K, Baweja A, Pai V, Kumar M. Diffusion tensor imaging in idiopathic inflammatory myopathies: A case– control study. Indian J Musculoskelet Radiol. 2024;6:104-10. doi: 10.25259/IJMSR_41_2024
Abstract
Objectives:
It was aimed to assess the potential of diffusion tensor imaging (DTI) in detecting muscle inflammation in individuals affected by idiopathic inflammatory myopathies (IIM) compared to healthy controls. Specifically, we investigated the impact of myositis-related inflammation on the diffusion of water molecules across the sarcolemma and its detectability through DTI.
Material and Methods:
This prospective cross-sectional observational study included 36 patients diagnosed with IIM ([based on clinical tests manual muscle testing [MMT8] and serological marker N-acetyl-cystein(NAC)-activated creatine kinase [CPK-NAC]) and 51 healthy controls. All participants underwent bilateral thigh magnetic resonance imaging (MRI) with a DTI protocol. For patients, three region of interests (ROI) (80 mm2 to 130 mm2) were delineated on the most affected muscle containing maximum signal intensity (edema), while in controls, the ROIs were drawn on the healthy vastus lateralis muscle and average of five readings were used for statistical analysis. Average apparent diffusion coefficient (ADC) value, fractional anisotropy (FA), and three eigenvalues: Maximum (λ1), middle (λ2), and minimum (λ3) were measured in all the subjects.
Results:
The average age for cases and controls was 33.08 ± 12.45 years and 40.70 ± 17.17 years, respectively, with no significant age or gender distribution differences. MMT8 scores averaged 103.33 ± 36.42, and CPK-NAC values averaged 4323.44 ± 6354.45 U/L. DTI analysis revealed significantly higher average ADC values in patients (2.07 ± 0.45) compared to controls (1.76 ± 0.26) with a P < 0.001. FA values showed no significant difference (0.38 ± 0.19 in patients vs. 0.33 ± 0.09 in controls, P = 0.094). The three eigenvalues in the patients were 2.76 ± 0.63, 2.35 ± 0.33, and 1.30 ± 0.29, respectively, and in controls were 2.37 ± 0.36, 1.72 ± 0.25, and 1.21 ± 0.27, respectively, with P = 0.001, <0.001, and 0.818, respectively.
Conclusion:
In individuals with IIM, disruptions in the sarcolemma lead to altered water molecule diffusion, detectable through DTI. The study demonstrated significant differences in average DTI ADC, maximum (λ1), and middle (λ2) eigenvalues between cases and controls (P < 0.001). Integrating DTI into routine myopathy MRI may enhance the differentiation between inflamed and normal muscles. Limitations included the absence of follow-up to observe treatment effects and the non-characterization of IIM into distinct subtypes.
Keywords
Idiopathic inflammatory myopathy
Diffusion tensor imaging
Magnetic resonance imaging
INTRODUCTION
Idiopathic inflammatory myopathies (IIM) are a rare and complex group of chronic, autoimmune muscle disorders that affect approximately 15–20 individuals per 100,000 globally.[1,2] The primary subtypes of IIM include dermatomyositis, polymyositis, and Inclusion body myositis, all of which manifest with progressive muscle weakness, inflammation, and varying degrees of disability.[3] While significant advancements have been made in understanding the pathophysiology of these conditions, the diagnosis of IIM remains a challenge. This is largely due to the heterogeneous nature of the disease, its variable clinical presentation, and its overlap with other neuromuscular disorders.[4]
Conventional diagnostic methods for IIM, such as muscle biopsy and serum muscle enzyme measurements, are frequently used but have notable limitations. Muscle biopsy, while considered the gold standard, is an invasive procedure that may not always yield definitive results, especially in cases where the muscle sample is not representative of the affected tissue.[5] Serum muscle enzyme measurements, another common diagnostic tool, can indicate muscle damage but are non-specific and may be elevated in a variety of other conditions, making it difficult to pinpoint the cause of muscle inflammation.[5]
Given these challenges, there has been a growing interest in utilizing imaging techniques as a non-invasive approach to diagnosing and monitoring IIM. Magnetic resonance imaging (MRI) has become a valuable tool in this regard. Traditional MRI techniques, which primarily provide detailed anatomical information, can reveal muscle edema, fatty infiltration, and atrophy hallmarks of muscle inflammation in IIM.[6,7] However, these conventional MRI methods have limitations, particularly in detecting early or subtle changes in muscle pathology. Early-stage disease or minimal inflammation may not be sufficiently captured by standard MRI, potentially delaying diagnosis and treatment.[6,7]
In recent years, diffusion tensor imaging (DTI), an advanced MRI technique, has emerged as a promising tool for assessing muscle pathology in IIM. DTI works by measuring the diffusion of water molecules within tissues, offering unique insights into muscle fiber integrity, directionality, and overall microstructure [Figure 1]. This technique is particularly valuable because it can detect microstructural changes in muscle tissue that are not visible on conventional MRI.[8,9] These microstructural changes may precede more overt signs of inflammation, making DTI a potentially powerful tool for the early detection of muscle involvement in IIM.[8,9]
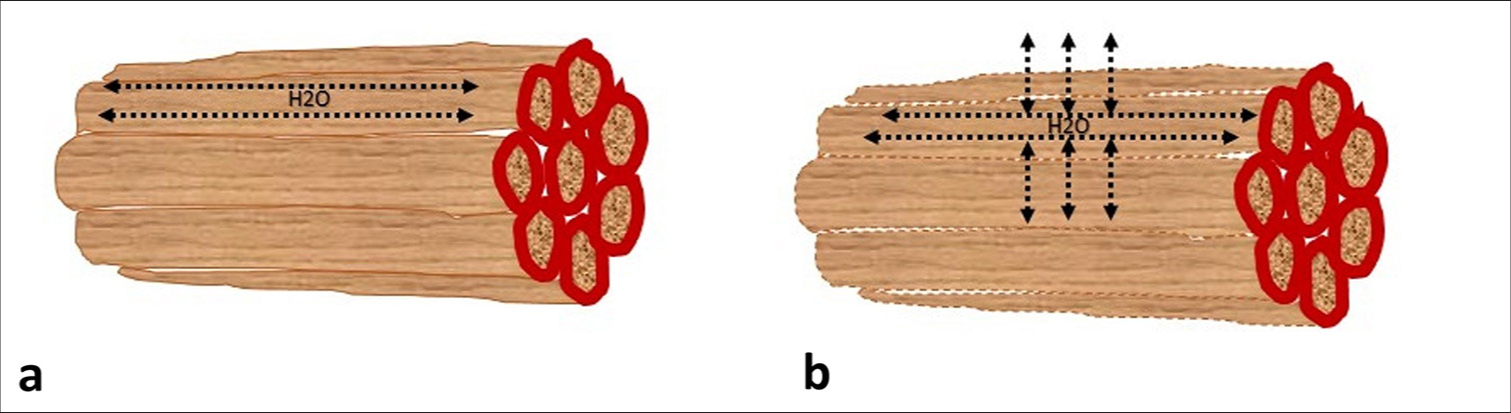
- Graphic showing normal muscle fibers (a) with the movement of water molecules along the length of myofibrils; and, muscle fibers affected by inflammation (b) showing breach of sarcolemma and movement of water molecules across the sarcolemma that can affect values of diffusion tensor imaging parameters.
This study aims to fill that gap by evaluating the potential of DTI in detecting muscle inflammation in individuals with IIM compared to healthy controls. If successful, DTI could offer a non-invasive and objective measure of muscle pathology, enhancing diagnostic accuracy and enabling better monitoring of disease progression. Ultimately, this could lead to improved patient outcomes by facilitating earlier diagnosis, more targeted treatments, and more effective monitoring of therapeutic responses in individuals with IIM.
MATERIAL AND METHODS
This prospective observational study with a cross-sectional study design was conducted in a tertiary care hospital for a period of 18 months from January 2023 to July 2024. “The study protocol was approved by the institutional research board and ethics committee and written informed consent was obtained from all the patients.” Inclusion criteria included all patients diagnosed with IIM based on clinical tests (manual muscle testing [MMT8]) and serological marker (CPK-NAC) referred to our department for MRI thighs. Age and gender-matched healthy controls with no complaints related to myositis were also included. Exclusion criteria included unwilling patients and contraindication to MRI (subjects with pacemakers, claustrophobia, cochlear, and metal implants).
Participants
A total of 38 consecutive patients with IIM diagnosed based on clinical tests MMT8 and serological marker (CPK-NAC) referred from the department of rheumatology and neurology were screened for inclusion and exclusion criteria. Two patients had contraindication to MRI and therefore excluded from the study. A total of 36 patients were included in the study. A total of 51 healthy age and gender-matched controls were also included. MRI of both thighs was performed in all the patients and healthy participants.
Imaging protocol
MRI of both thighs was performed on a 3T magnetic resonance scanner (General Electric Healthcare Discovery 750W) using a 16-channel torso body coil. Initially, axial T1-weighted fast spin echo (FSE) (repetition time/echo time [TR/TE] 440/10 ms) and T2-weighted FSE with fat suppression (TR/TE 3800/70 ms) were obtained with flip angle 111°, slice thickness 4 mm, field of view (FOV) 42 cms, number of excitations (NEX) 1 and slice gap 1.5 mm. DTI sequences in the axial plane were obtained using an echo planar imaging sequence with 15 gradient directions. The acquisition parameters for DTI were as follows: b-value of 500 s/cm2; TR/TE, 6000/1 ms; flip angle 90°, slice thickness 6 mm, matrix size 192 × 160, and FOV 38 cms. Each DTI acquisition had an imaging time of about 3.18 min, and the total scan time for each subject was around 15 min.
Image analysis
A musculoskeletal radiologist, with 5 years of experience in the field, conducted an analysis of the MRI images. The reader was kept unaware of the clinical details of the patients. The measurement of DTI parameters was performed on an offline workstation (ADW 4.4; GE Healthcare). Three circular or oval regions of interests (80 mm2 to 130 mm2) were delineated on the most affected muscle containing maximum signal intensity (edema) in cases and on the mid-slice of healthy vastus lateralis muscle in controls and average of the reading was used for calculation. The DTI measurements included average ADC, fractional anisotropy (FA), and three eigenvalues: Maximum (λ1), middle (λ2), and minimum (λ3). ADC was quantified to assess the extent of diffusion occurring across the sarcolemma, while FA and eigenvalues were measured to quantify the extent of anisotropy. Figure 2 is showing a flow chart depicting the study methodology.
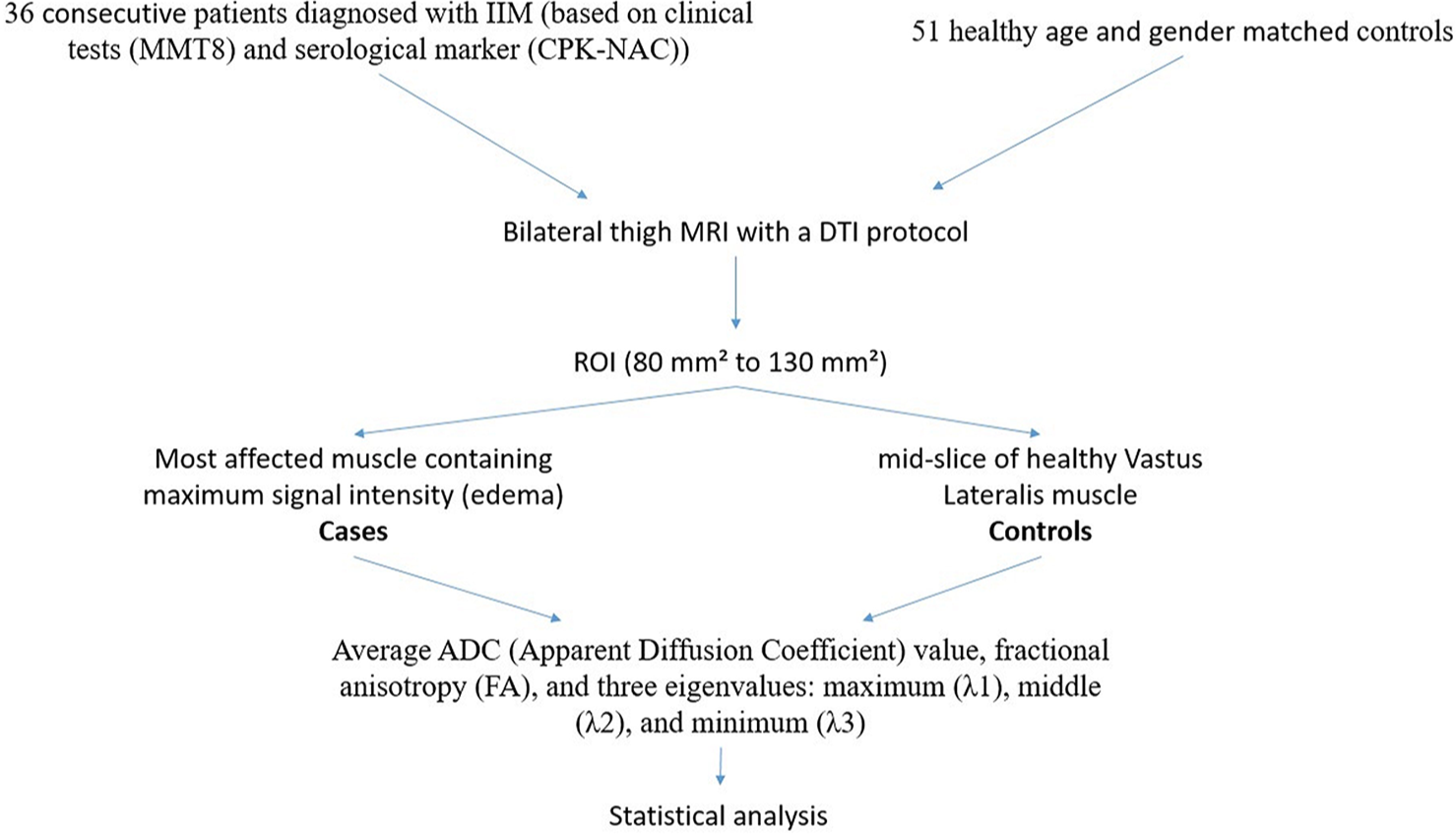
- Flow chart depicting study methodology. IIM: Idiopathic inflammatory myopathies, CPKNAC: N-acetyl-cystein-(NAC)-activated creatine kinase, MMT8: Manual Muscle Testing 8, DTI: Diffusion tensor imaging, ROI: Region of interest, ADC: Apparent diffusion coefficient.
Statistical analysis
Clinical and subject-related variables were: Age (in years), gender (male/female), MMT8 score, and CPK-NAC (U/L) values. DTI-related variables comprised average ADC, FA, and three Eigenvalues. On entering data into an MS Excel spreadsheet, Statistical Package for the Social Sciences v23 (IBM Corp.) was employed for data analysis. Descriptive statistics utilized mean with standard deviations, continuous variables were presented as median with interquartile range, and categorical variables were expressed as frequencies with percentages. The association between different variables was examined using an independent sample t-test, Wilcoxon Test, Chi-squared test, and Fisher’s Exact test as appropriate. Linear correlations between two continuous variables were determined using Pearson’s correlation and Spearman’s correlation. A P < 0.05 was considered statistically significant.
RESULTS
The study included patients with IIM and healthy controls, with an average age of 33.08 ± 12.45 years for the case group and 40.70 ± 17.17 years for the control group. There were no significant differences in age or gender distribution between the two groups, indicating a well-matched study population.
Clinical assessment using MMT8 yielded an average score of 103.33 ± 36.42 in the patient group, reflecting the degree of muscle weakness typically associated with the condition. In addition, the serological marker CPK-NAC, which is often elevated in muscle inflammation, showed a markedly high average value of 4323.44 ± 6354.45 U/L in the patient group. These tests were not done in the control group.
DTI analysis provided further insights into muscle pathology. The average ADC values were significantly higher in the patient group (2.07 ± 0.45) compared to the controls (1.76 ± 0.26), with a P < 0.001, suggesting increased muscle water diffusion in IIM patients, which is indicative of muscle inflammation and edema. In contrast, FA values, which measure the directionality of water diffusion, showed no significant difference between the two groups (0.38 ± 0.19 in patients vs. 0.33 ± 0.09 in controls, P = 0.094).
Further analysis of the three eigenvalues, which represent the diffusion in different directions within the muscle fibers, revealed significant differences in two of the eigenvalues. In patients, the eigenvalues were 2.76 ± 0.63, 2.35 ± 0.33, and 1.30 ± 0.29, while in controls, they were 2.37 ± 0.36, 1.72 ± 0.25, and 1.21 ± 0.27. The first two eigenvalues showed significant differences with P = 0.001 and <0.001, respectively, suggesting alterations in muscle fiber integrity and structure in IIM patients, while the third eigenvalue did not show a significant difference (P = 0.818). Table 1 is showing summary of results. Figures 3 and 4 are showing DTI analysis in a case and control, respectively.
| Parameters | Group | P-value | |
|---|---|---|---|
| Case (n=36) | Control (n=51) | ||
| Age (Years) | 33.08±12.45 | 40.70±17.17 | 0.333[1] |
| Gender | |||
| Male | 13 (36.1%) | 17 (33.3%) | 0.865[2] |
| Female | 23 (63.8%) | 34 (66.6%) | |
| DTI: ADC (e–09) Avg*** | 2.07±0.45 | 1.76±0.26 | <0.001[1] |
| DTI: FA Avg | 0.38±0.19 | 0.33±0.09 | 0.094[1] |
| DTI: Max Eigen value e–09 (Avg)*** | 2.76±0.63 | 2.37±0.36 | 0.001[1] |
| DTI: Middle Eigen value e-09 (Avg)*** | 2.35±0.33 | 1.72±0.25 | <0.001[1] |
| DTI: Minimum Eigen value e–09 (Avg) | 1.30±0.29 | 1.21±0.27 | 0.818[1] |
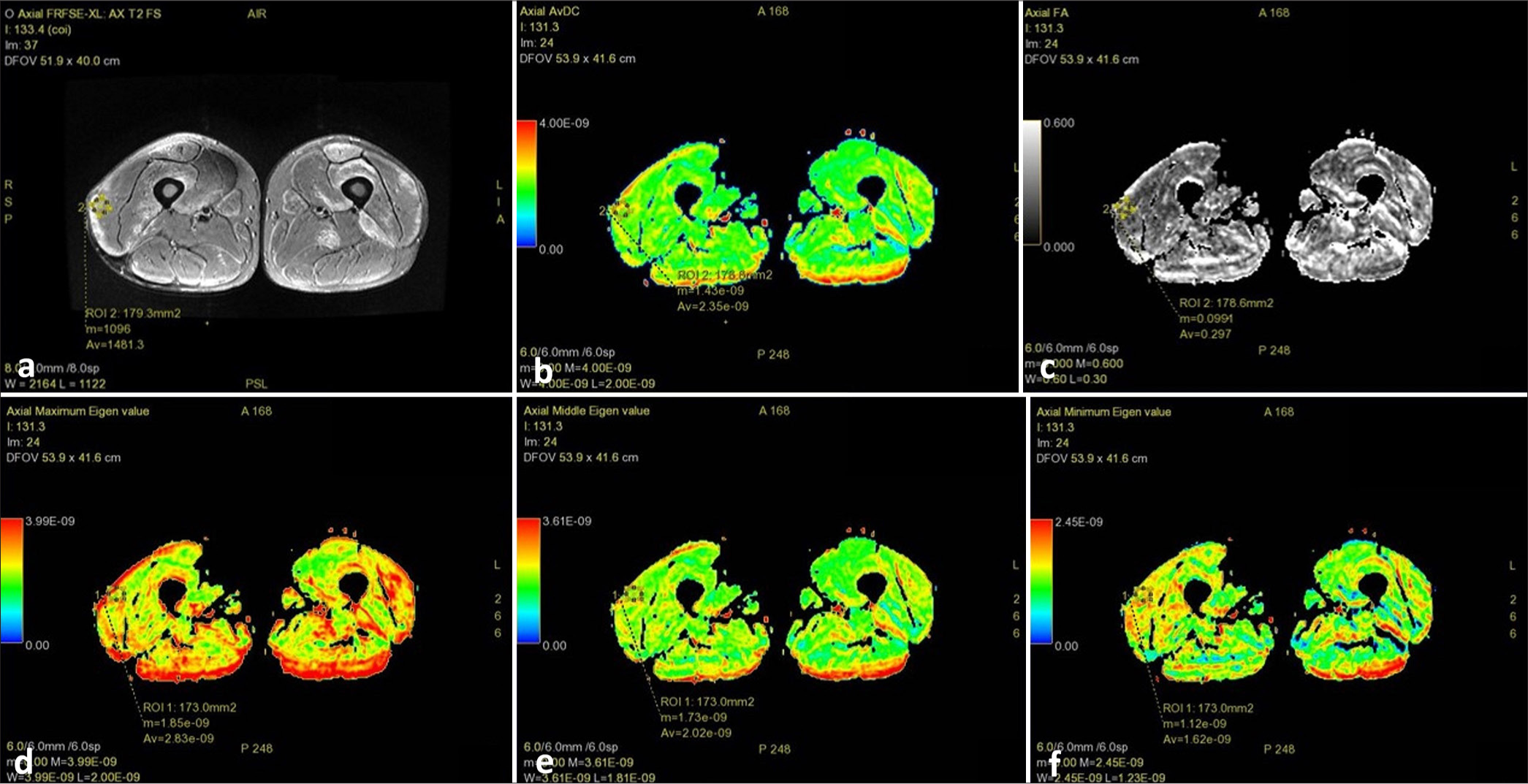
- Magnetic resonance imaging thigh of a patient with inflammatory myopathy showing: (a) Axial T2 fat saturated image showing muscle edema with a slice of the muscle selected that contained maximum signal intensity to place region of interest; (b) diffusion tensor imaging apparent diffusion coefficient (average 2.35 e–09); (c) fractional anisotropy (average 0.297); (d) λ1 value (average 2.83 e–09); (e) λ2 value (average 2.02 e–09); and, (f) λ3 value (average 1.62 e–09).
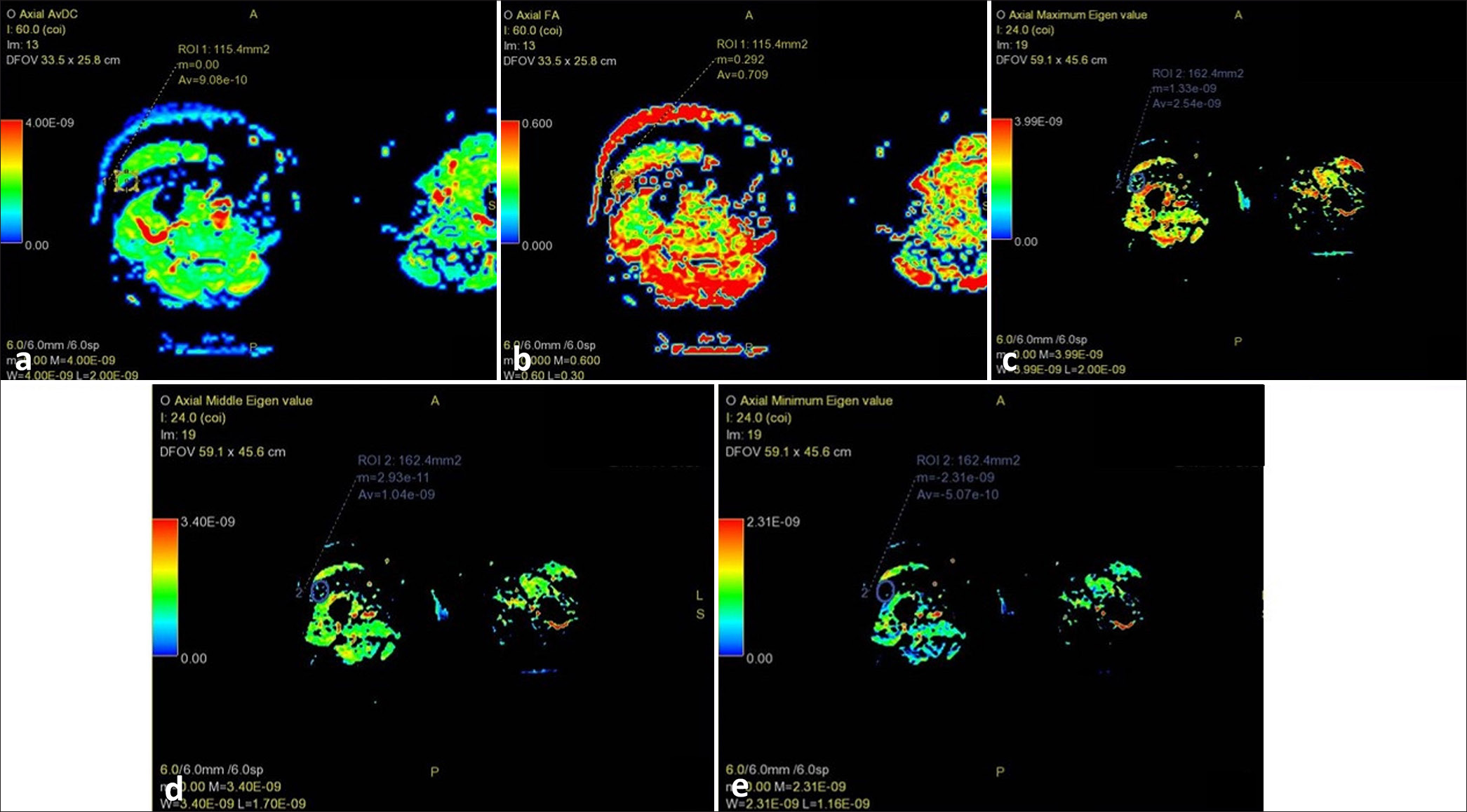
- Magnetic resonance imaging thigh of a healthy control showing selection of mid-slice of vastus lateralis for placing region of interest: (a) diffusion tensor imaging apparent diffusion coefficient (average 9.08 e–10); (b) fractional anisotropy (average 0.709); (c) λ1 value (average 2.54 e–09); (d) λ2 value (average 1.04 e–09); and, (e) λ3 value (average 5.07 e–10).
DISCUSSION
This study builds on the growing body of evidence supporting the use of DTI in the evaluation of IIM. In individuals with IIM, disruptions in the sarcolemma lead to altered water molecule diffusion, detectable through DTI. This study demonstrated significant differences in average DTI ADC, maximum (λ1), and middle (λ2) eigenvalues between cases and controls (P ≤ 0.001).
Ai et al. conducted a comparative analysis of DTI parameters between IIM patients and healthy controls, reporting higher ADC, FA, and eigenvalues in the patient group. Their findings are consistent with our results, where increased ADC and certain eigenvalues in IIM patients reflect microstructural changes in muscle tissue, likely due to inflammation and muscle fiber disarray. The elevated FA values, which indicate greater diffusion directionality, may be related to the complex muscle fiber alterations occurring in inflamed tissue.[10]
The utility of DTI in distinguishing IIM patients from healthy controls was further demonstrated by Sigmund et al., who found significant differences in DTI parameters between dermatomyositis patients and controls.[11] Our study corroborates these findings.
Wang et al. evaluated the use of simultaneous multislice (SMS) accelerated DTI for assessing thigh muscles in patients with myositis. The SMS technique allowed for faster image acquisition while maintaining high image quality, making it feasible to assess muscle pathology more efficiently. The study demonstrated that SMS-accelerated DTI could effectively capture muscle abnormalities in myositis, offering a promising tool for the non-invasive evaluation of muscle inflammation and damage in clinical practice.[12]
Some other studies evaluated the role of Diffusion-weighted imaging (DWI) in myositis such as that by Qi et al., who demonstrated that inflamed muscles in myositis patients exhibited elevated ADC and diffusion coefficient (D) values, with concomitantly lower perfusion fraction (f) values when compared to unaffected muscles. This underscores the ability of DWI to sensitively detect alterations in muscle water diffusion related to inflammation, offering a non-invasive biomarker for disease activity.[13] Similarly, the work by Faruch et al. highlighted a positive correlation between mean ADC values and muscle edema grading, further reinforcing the association between increased diffusion metrics and the presence of muscle pathology. Interestingly, their study also revealed that even low-grade muscle edema, which might not be readily apparent on conventional short tau inversion recovery sequences, could be detected by high ADC values.[14] This suggests that DWI may offer enhanced sensitivity over traditional MRI techniques in identifying early or subtle changes in muscle tissue, which is particularly valuable in the context of IIM where early intervention is critical.
Meyer et al. conducted two studies; one exploring the relationship between ADC values from MRI and electromyography (EMG) parameters in patients with myositis, which found significant associations between ADC values and certain EMG parameters, suggesting that ADC could potentially serve as a biomarker for muscle involvement in myositis; and, another study compared conventional ADC analysis with histogram analysis derived from ADC values in assessing myositis which indicated that histogram analysis is more sensitive in reflecting serological parameters associated with myositis, highlighting its potential for improved diagnostic accuracy in this condition.[15,16]
This study has several limitations that should be acknowledged. First, the sample size was relatively small, comprising 36 patients with IIM and 51 healthy controls, which may limit the generalizability of the findings. In addition, the study employed a cross-sectional design, capturing data at a single point in time, thereby restricting the ability to assess changes in DTI parameters over time or establish causality. The lack of a longitudinal follow-up limits the evaluation of DTI’s potential as a tool for monitoring disease progression or treatment response. Moreover, muscle biopsy, the current gold standard for IIM diagnosis, was not used as a reference standard in this study, limiting the validation of DTI findings. The subjective nature of the ROI selection during image analysis introduces potential bias and may affect the reproducibility of the results. No interobserver variability was measured. Future studies can try to explore DTI values in apparently normal-appearing muscles and see if early changes can be detected. Finally, the heterogeneity of IIM subtypes among the patient population was not addressed, which may have influenced the DTI findings and the overall study conclusions.
CONCLUSION
The results from our study and the referenced literature collectively highlight the potential of DTI as valuable tools for assessing muscle inflammation in IIM. By offering noninvasive and objective measures of muscle pathology, DTI can significantly enhance diagnostic accuracy and potentially guide therapeutic decision-making in clinical practice. Further research with larger cohorts and longitudinal designs is warranted to validate these findings and explore the full clinical utility of DTI in IIM.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, Medicare supplemental insured, and Medicaid enrolled populations: An administrative claims analysis. BMC Musculoskelet Disord. 2012;13:103.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic inflammatory myopathies: Clinical aspects. Baillieres Best Pract Res Clin Rheumatol. 2000;14:37-54.
- [CrossRef] [PubMed] [Google Scholar]
- Polymyositis, dermatomyositis, and inclusion-body myositis. N Engl J Med. 1991;325:1487-98.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatomyositis and polymyositis: A guide for the clinician. Nat Rev Rheumatol. 2009;5:691-701.
- [Google Scholar]
- Autoimmune myopathies: Autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol. 2011;7:343-54.
- [CrossRef] [PubMed] [Google Scholar]
- MRI scoring methods used in evaluation of muscle involvement in patients with idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2017;29:623-31.
- [CrossRef] [PubMed] [Google Scholar]
- An efficacy analysis of whole-body magnetic resonance imaging in the diagnosis and follow-up of polymyositis and dermatomyositis. PLoS One. 2017;12:e0181069.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of skeletal muscle diffusion tensor imaging. Skeletal Radiol. 2023;52:1639-1649.
- [CrossRef] [PubMed] [Google Scholar]
- Role of diffusion tensor imaging in the evaluation of muscle inflammation: A systematic review. Eur J Radiol. 2016;85:923-31.
- [Google Scholar]
- Diffusion tensor imaging in evaluation of thigh muscles in patients with polymyositis and dermatomyositis. Br J Radiol. 2014;87:20140261.
- [CrossRef] [PubMed] [Google Scholar]
- MRI assessment of the thigh musculature in dermatomyositis and healthy subjects using diffusion tensor imaging, intravoxel incoherent motion and dynamic DTI. Eur Radiol. 2018;28:5304-15.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous multislice accelerated diffusion tensor imaging of thigh muscles in myositis. Am J Roentgenol. 2018;211:861-6.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted imaging of inflammatory myopathies: Polymyositis and dermatomyositis. J Magn Reson Imaging. 2008;27:212-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted magnetic resonance imaging is useful for assessing inflammatory myopathies. Muscle Nerve. 2019;59:555-60.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between apparent diffusion coefficient and electromyography parameters in myositis-A preliminary study. Brain Behav. 2018;8:e00958.
- [CrossRef] [PubMed] [Google Scholar]
- Histogram analysis derived from apparent diffusion coefficient (ADC) is more sensitive to reflect serological parameters in myositis than conventional ADC analysis. Br J Radiol. 2018;91:20170900.
- [CrossRef] [PubMed] [Google Scholar]






