Translate this page into:
To study magnetic resonance imaging findings and inflammatory markers in inflammatory sacroiliitis

*Corresponding author: Dr. Kunwar Pal Singh, Professor, Department of Radiodiagnosis, Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India. kpsdhami@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh K, Arora M, Arora V, Singh A, Kaur S. To study magnetic resonance imaging findings and inflammatory markers in inflammatory sacroiliitis. Indian J Musculoskelet Radiol. 2023;5:113-21. doi: 10.25259/IJMSR_20_2023
Abstract
Objectives:
The objectives of the study are to determine magnetic resonance imaging (MRI) findings in inflammatory sacroiliitis and assign scores and grades to it and to determine and correlate erythrocyte sedimentation rate, C-reactive protein (CRP), and human leukocyte antigen-B27 (HLA-B27) in various grades of sacroiliitis.
Material and Methods:
An observational cross-sectional study was conducted on 30 patients who clinically presented with features of sacroiliitis and underwent an MRI of sacroiliac joint (SIJ). Various inflammatory and structural findings on MRI were used to do Spondyloarthritis Research Consortium of Canada scoring and grading. Then inflammatory markers including erythrocyte sedimentation rate, CRP, and HLA-B27 were studied in various grades of sacroiliitis.
Results:
Inflammatory sacroiliitis affects commonly the age group of 21–40 years. Periarticular edema was the most common finding seen with the iliac aspect more commonly involved. The majority of the subjects were graded moderate (50%). Values of erythrocyte sedimentation rate and CRP levels were raised whereas HLA-B27 was positive in 9 patients (30%) of inflammatory sacroiliitis.
Conclusion:
Inflammatory sacroiliitis presents with a chief complaint of low back ache. MRI helps to grade it into mild, moderate, and severe. STIR is the most sensitive sequence for the detection of bone marrow edema with bilateral symmetrical involvement but the iliac bone of SIJ is more involved than the sacral side. Contrast-enhanced sequences and diffusion images add no significant statistical role in the diagnosis of bone marrow edema. Inflammatory laboratory parameters were increased in higher grades of sacroiliitis. HLA-B27, although not specific to inflammatory sacroiliitis, increases in higher grades of sacroiliitis.
Keywords
Sacroiliitis
Human leukocyte antigen B-27
Magnetic resonance imaging
C-reactive protein
Erythrocyte sedimentation rate
INTRODUCTION
Sacroiliitis is a component of spondyloarthropathies such as ankylosing spondylitis (AS), psoriatic arthritis, and Reiter syndrome. It is also seen in tuberculosis, gout, rheumatoid arthritis, enteropathic arthropathy, and pyogenic arthritis.[1] These diseases predominantly affect the axial skeleton, causing pain and stiffness, making the diagnosis of sacroiliitis in its early stage difficult based on clinical features. Radiological evaluation plays a major role in the diagnosis of sacroiliitis.[2,3] Magnetic resonance imaging (MRI) is being extensively used to evaluate intra-articular abnormalities. MRI is thus one of the crucial tools for diagnosing sacroiliitis because it allows the assessment of acute inflammatory changes. Besides this, it can also detect inflammatory changes even in advanced stages in which ankylosis of the sacroiliac joint (SIJ) has taken place.[4] Knowing the anatomy of the SIJ is important for the correct interpretation of the findings. The SIJ has two well-differentiated parts. The first is a ventral-lower part with a cartilaginous articulation consistent with a symphysis and a dorsal-upper part, a syndesmosis with very irregular edges.[5]
Multiple anatomical variants of the SIJ have been described in the literature. Different factors have been suggested regarding their etiology, including congenital or hereditary factors, as well as the influence of mechanical stress. SIJ variants are variations in the morphology of the osseous structures of the sacrum and the ilium. As computed tomography (CT) depicts the bone well, most studies describing these variants have been conducted on CT. Variations in cartilaginous as well as ligamentous parts exist. Variations in the cartilaginous part include an unfused ossification center, with a separate often triangular osseous structure anterosuperiorly to the SIJ, focal dysmorphic sacrum, formed by a prominent ridge of the posterior part of the sacral surface of the joint, protruding into the iliac bone, and isolated synostosis with focal bony bridging. The variations in the ligamentous part include bipartite iliac bony plate, with a division in the posterior part of the iliac part of the SIJ, accessory joint posterior to the cartilaginous part of the joint, iliosacral complex with a prominent convex notch and a corresponding sacral groove, semicircular defect where there is a round defect in the sacrum and sometimes also in the overlying ilium, crescent iliac bony plate where the normal overall convex ilium is concave, with or without bulging of the sacral surface.[6]
The most reliable, prevalent, and diagnostic feature of active sacroiliitis on MRI is bone marrow edema. Besides this, SIJ erosions are also to be considered as these add specificity to the diagnosis of inflammatory sacroiliitis. The Spondyloarthritis Research Consortium of Canada (SPARCC) score is one of the most popular ones for scoring sacroiliitis.[7] The SPARCC scoring method is based on the presence of active inflammatory lesions in the synovial portion of the SIJs depicted on 6 consecutive coronal slices. The MR appearances may be compatible with inflammatory sacroiliitis but for a diagnosis of spondyloarthropathy to be made, the clinical and serological criteria should also be considered.[8,9]
In addition to sacroiliitis, there is inflammatory involvement of the entheses, which are junctional areas between bone and tendons, fascia, ligaments, or capsules. Enthesopathy is a prominent clinical feature in patients with spondyloarthritis, usually affecting the axial skeleton and SIJs. The initial inflammatory lesion seems to be responsible for the subsequent finding of erosions in the subchondral bone that is filled with subacute or chronic inflammatory tissue. These erosive lesions are usually healed by new bone formation, which tends to fill the initial bony defect and form a bridge between the deeper bone and the end of the ligament, creating a new enthesis. Such entheses have a high level of metabolic activity, have an abundant nerve supply, and are responsible for the pain.[10]
Elevation of serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels has been demonstrated as a risk factor for heart failure, acute stroke, infective endocarditis, and diabetic nephropathy. These laboratory values are also raised in different grades of sacroiliitis. We assumed that serum CRP and ESR could be a blood biomarkers in spondyloarthropathy (SpA).[11]
Human leukocyte antigen B27 (HLA-B27) is a polymorphic form of the HLA-B molecule that is found in only 8% of the general population worldwide. The most frequent subtypes (HLA-B*2705, HLA-B*2702, HLA-B*2704, and HLA-B*2707) are commonly associated with a risk for spondyloarthritis. Thus, the positivity of HLA-B27 can also be correlated with grades of inflammatory sacroiliitis.[12,13]
MATERIAL AND METHODS
Patient cohort
An observational cross-sectional study was conducted on thirty subjects of the age group of 18–60 years, who attended outpatient department or were admitted to the hospital with clinical suspicion of inflammatory sacroiliitis. MRI of the SIJs was done on 1.5 Tesla Philips Gyroscan Achieva Dstream after taking informed written consent. MRI findings were used to grade sacroiliitis and correlated with the laboratory findings.
Patient inclusion criteria
Patients between 18 and 60 years of age and either gender
Patients with clinical suspicion of pain/inflammation involving the SIJ.
Imaging
The study was conducted from April 2021 to October 2022 after getting approval from the ethical committee of the institute. Informed written consent was taken from all the patients before inclusion in the study. After taking the informed, written consent of each patient clinical history was recorded and an MRI of the SIJ was done.
MRI technique
All the patients were examined under a 1.5-Tesla MRI scanner (Philips Achieva Dstream)
Findings seen at the SIJ were interpreted, graded, and correlated with the clinical history and significant laboratory markers.
MR imaging for SIJ required coronal oblique imaging, which was taken parallel to the long axis of the sacrum. However, doing the imaging in two perpendicular (coronal and axial oblique) planes added to the sensitivity for the changes in the ligamentous portion of the SIJ. The entire sacral bone was imaged from its anterior to its posterior border, requiring at least 10–12 sections. Structural changes were visualized on coronal and axial oblique fast spin-echo T1-weighted sequences. Coronal and axial oblique short-time inversion recovery (STIR) or fat-saturated (FS) fast spin-echo T2-weighted sequences were used to detect acute inflammatory changes [Table 1].
| Sequences | TR (msec) | TE (msec) | THK/Slice GAP (mm) | FOV (mm)2 | NSA | IT (msec) |
|---|---|---|---|---|---|---|
| STIR OBLIQUE CORONAL | 4000–5000 | 70–80 | 3.5/0.3 | 380 | 2 | 145–165 |
| T1W OBLIQUE CORONAL | 400–500 | 12–16 | 3.5/0.3 | 380 | 2 | |
| T2FS AXIAL | 3500–4000 | 90–120 | 3.5/0.3 | 300 | 2 | |
| T1W AXIAL | 400–500 | 12–16 | 3.5/0.3 | 300 | 2 | |
| DWI AXIAL (b-value 0,300,600) | 5000–6000 | 100–110 | 3.5/0.3 | 300 | 2 | |
| FST1 AXIAL (without/with contrast) | 400–500 | 12–16 | 3.5/0.3 | 300 | 2 |
DWI: Diffusion-weighted imaging, FS: Fat-saturated, STIR: Short-time inversion recovery, T1W: T1-weighted, TR: Repetition time, TE: Time to echo, THK: Slice thickness, GAP: Slice gap, FOV: Field of view, NSA: Number of excitation, IT: Inversion time, T2FS: T2-Fat-saturated
Active inflammation in the SIJs was demonstrated with FS fast spin-echo T1-weighted sequences after the administration of gadolinium-based contrast material. The intravenous administration of gadolinium-based contrast material also helped in detecting subtle osteitis or bone marrow edema (BME) evaluation of enthesitis and capsulitis.
Diffusion-weighted imaging was done using b values (0, 300, and 600). Apparent diffusion coefficient (ADC) maps were derived automatically on a voxel-by-voxel basis. ADC was calculated from diffusion-weighted images. Region of interest was drawn in the involved area and evaluated.
Imaging analysis
Scoring of inflammatory lesions at SIJ was done by the SPARCC scoring method for active inflammatory lesions in the SIJ using a T2-weighted sequence that incorporates the suppression of normal marrow fat signals. Each SIJ was divided into four quadrants: Upper iliac, lower iliac, upper sacral, and lower sacral. The presence of an increased signal on short-time inversion recovery (STIR) sequence in each of these four quadrants was scored on a dichotomous basis, where 1 represents an increased signal and 0 represents a normal signal. The maximal score for an abnormal signal in the two SIJs of one coronal slice was therefore 8. Joints that included a lesion exhibiting an intense signal were given an additional score of 1 per slice to demonstrate this feature. Similarly, each joint that included a lesion demonstrating a continuous increased signal of a depth of 1 cm from the articular surface was also given an additional score of 1. These additional measures bring the maximal score for a single coronal slice to 12. The scoring was repeated in each of the six consecutive coronal slices leading to a maximal score of 72.
Scoring of structural lesions at SIJs included the SPARCC SIJ structural scoring including the structural lesions of fatty marrow, bone erosions, ankylosis, and SIJ space.
Inflammatory markers including erythrocyte sedimentation rate, CRP, and HLA-B27 were done in these patients, and values were correlated to different grades of sacroiliitis.
A blood sample (3 mL) for erythrocyte sedimentation rate was collected in a lavender vial and sent to the pathology department for calculation using the Westergren method with the normal biological reference of 4–20 mm/h. The serum sample (4 mL) for CRP was collected in the red vial and sent to the biochemistry department for calculation using the particle-enhanced turbidimetric immunoassay technique. The biological reference value for the serum sample is 0–5.0 mg/L. HLA-B27 positivity or negativity was calculated from the blood sample taken in a lavender top container using flow cytometry.
Statistical analysis
The collected data were transformed into variables, coded, and entered in Microsoft Excel. Data were analyzed and statistically evaluated using the SPSS-PC-25 version.
The normal distribution of different parameters was tested by the Shapiro–Wilk normality test. Quantitative data were expressed in mean ± standard deviation or median with interquartile range and depends on normality difference between the mean of two groups were compared by Mann–Whitney U-test. Qualitative data were expressed in frequency and percentage and statistical differences between the proportions were tested by Chi-square test or Fisher’s exact test. Spearman’s correlation coefficient was used to see a correlation between SPARCC level with CRP and ESR level. P < 0.05 was considered statistically significant.
RESULTS
In the present study of 30 patients with sacroiliitis, almost all presented with chief complaints of low back aches. Other chief complaints were uveitis, swelling of hands, and backache radiating to the legs. Out of 30 patients with sacroiliitis, majority of the patients (36.7%) were in the age group of 21–40 years [Table 2]. The second most common age group was >40 years (16.7%). The youngest subject was aged 18 years and the eldest was 48 years with a mean age of 32.47 ± 8.14 years. Male and female populations contributed 17 and 13 cases, respectively, in the study. On MRI, BME (100%) was the most common imaging finding in patients presenting with sacroiliitis [Tables 3 and 4]. Sacral edema was found in 21 subjects (70%) with unilateral and bilateral involvement seen in 12 (40%) and 9 (30%) subjects, respectively, [Figure 1]. Iliac side edema was found in 30 subjects (100%) with unilateral and bilateral involvement seen in 9 (30%) and 21 (70%) subjects, respectively [Table 5]. Bilateral involvement of other findings including fatty marrow changes, bone erosions, ankylosis, and reduced joint space was found in 11 (36.7%), 1 (3.3%), 1 (3.3%), and 0 (0%) subjects, respectively [Figure 2]. SIJ space was narrowed to 07 patients. The majority of the patients’ joint space was maintained. The iliac aspect of SIJ was involved in 30 patients, sacral aspect in 21 patients while both iliac and sacral aspects were involved in 14 patients on the right side and 15 patients on the left side patients [Figure 3]. There was almost symmetrical involvement of the right and left side involving SIJ of all the features of inflammatory sacroiliitis with the iliac side (100%) involved more frequently than the sacral side (70%). The majority of the patients with inflammatory sacroiliitis were graded moderate (50%) according to the SPARCC scoring system [Table 6]. Mild and severe grades were 43.3% and 6.7%, respectively. There was no statistically significant role of contrast and diffusion images on STIR with a depth of bone marrow edema almost similar in the two of inflammatory sacroiliitis [Table 7]. STIR coronal sequence is the most sensitive sequence for inflammatory activity. Values of ESR and CRP levels were raised in inflammatory sacroiliitis with mean values of ESR and CRP being 12.93 ± 4.66 mg/L and 28.93 ± 8.75 mm/h, respectively [Table 8]. Their values correlated with the SPARCC grading system with higher values in higher scores of sacroiliitis. HLA B27 was positive in 9 (30%) of the patients with inflammatory sacroiliitis, but there was a positive association between HLA B27 positivity and higher grades of sacroiliitis [Tables 9-11].
| Age group | No. | % |
|---|---|---|
| Up to 20 years | 3 | 10.0 |
| 21–30 years | 11 | 36.7 |
| 31–40 years | 11 | 36.7 |
| 41–50 years | 5 | 16.7 |
| Total | 30 | 100.0 |
| MRI findings | Absent (%) | Unilateral (%) | Bilateral (%) |
|---|---|---|---|
| Sacral edema | 9 (30.0) | 12 (40.0) | 9 (30.0) |
| Iliac edema | 0 (0.0) | 9 (30.0) | 21 (70.0) |
| Fatty marrow | 15 (50.0) | 4 (13.3) | 11 (36.7) |
| Bone erosion | 29 (96.7) | 0 (0.0) | 1 (3.3) |
| Ankylosis | 29 (96.7) | 0 (0.0) | 1 (3.3) |
| Reduced joint space | 23 (76.7) | 7 (23.3) | 0 (0.0) |
MRI: Magnetic resonance imaging
| MRI findings | Right side (%) | Left side (%) | P-value |
|---|---|---|---|
| Sacral edema | 14 (46.7) | 16 (53.3) | 0.60 |
| Iliac edema | 25 (83.3) | 26 (86.7) | 1.0 |
| Fatty marrow | 12 (40.0) | 14 (46.7) | 0.60 |
| Bone erosion | 1 (3.3) | 1 (3.3) | 1.0 |
| Ankylosis | 1 (3.3) | 1 (3.3) | 1.0 |
| Reduced joint space | 3 (10.0) | 4 (13.3) | 1.0 |
MRI: Magnetic resonance imaging
| Sacral involvement | Iliac involvement | P-value | |
|---|---|---|---|
| Number of subjects with involvement | 21 (70%) | 30 (100%) | 0.001 |
| Grade of sacroiliitis | No. | % |
|---|---|---|
| Mild (<24) | 13 | 43.3 |
| Moderate (24–48) | 15 | 50.0 |
| Severe (>48) | 2 | 6.7 |
| Non- contrast MR |
Contrast MR | P-value | Diffusion | P-value | |
|---|---|---|---|---|---|
| Mean depth | 4.53±2.19 | 4.63±2.32 | 0.86 | 4.67±2.41 | 0.82 |
MR: Magnetic resonance, STIR: Short-tau inversion recovery
| CRP | ESR | |
|---|---|---|
| Mean±SD | 12.93±4.66 | 28.93±8.75 |
| Median | 12.800 | 29.00 |
| IQR | 10–16.4 | 23.5–34.5 |
| Minimum | 4.0 | 12 |
| Maximum | 22.0 | 48 |
CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate, SD: Standard deviation, IQR: Interquartile range
| HLA B-27 | No. | % |
|---|---|---|
| Negative | 21 | 70.0 |
| Positive | 9 | 30.0 |
HLA-B27: Human leukocyte antigen-B27
| r-value | P-value | |
|---|---|---|
| CRP level | 0.62 | <0.001 |
| ESR level | 0.85 | <0.001 |
SPARCC: Spondyloarthritis Research Consortium of Canada, CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate
| Negative | Positive | P-value | |
|---|---|---|---|
| SPARCC score | 24.52±6.03 | 39.89±7.72 | <0.001 |
SPARCC: Spondyloarthritis Research Consortium of Canada, HLA-B27: Human leukocyte antigen-B27
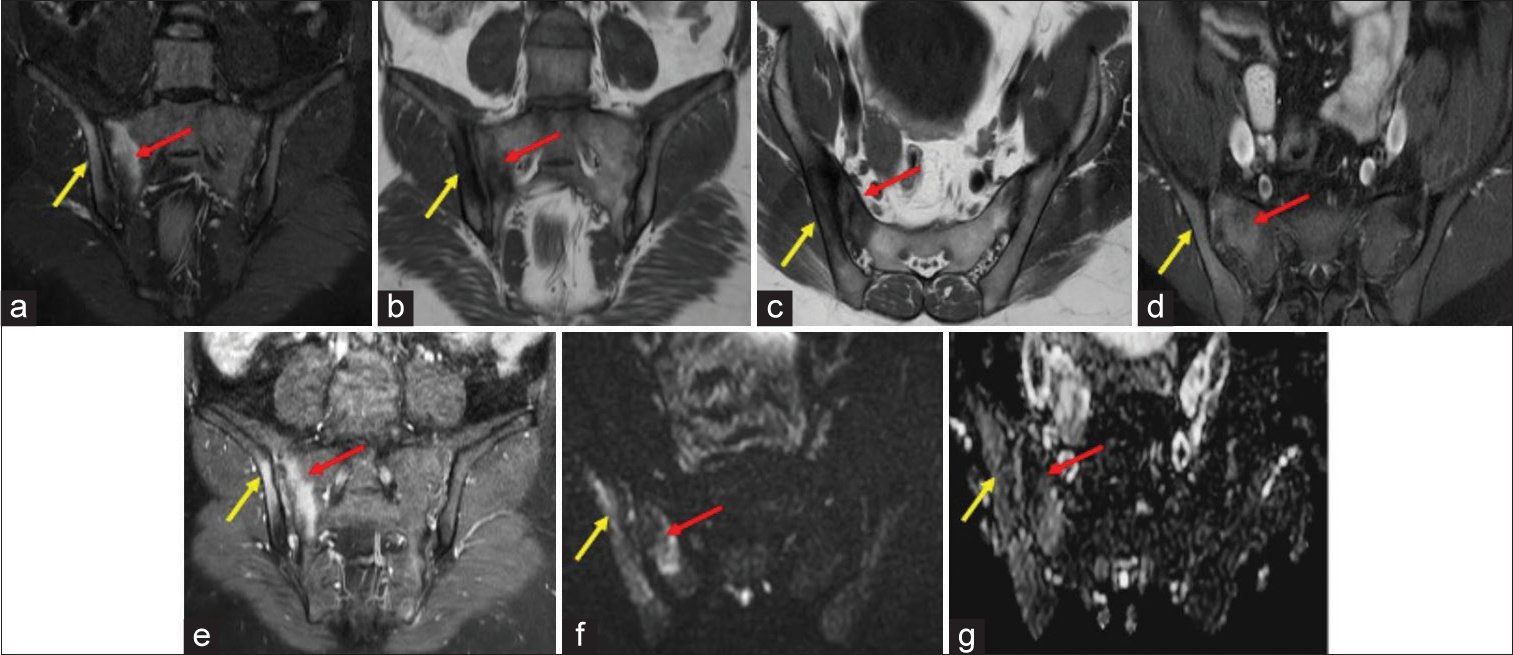
- Magnetic Resonance images of the sacroiliac joints of a 47 years old male patient with positive Human leukocyte antigen-B27 and raised C Reactive Protein (16.6 mg/L) and erythrocyte sedimentation rate (42 mm/hr). (a) Oblique coronal Short-tau inversion recovery image showing hyperintensities along the periarticular surface of right sacroiliac joints involving both sacral (red arrow) and iliac (yellow arrow) sides. T1 Weighted images in oblique coronal (b) and axial (c) planes showing hypointensity as shown by red (b) and yellow (c) arrows. T1Weighted Fat Saturatetd post contrast images in axial (d) and oblique coronal (e) planes showing enhancement along the periarticular surface of right SIJ involving both sacral (red arrow)and iliac (yellow arrow) sides. (f) Diffusion weighted sequence in oblique coronal plane showing hyperintensity along the periarticular surface of right SIJ involving both sacral (red arrow)and iliac (yellow arrow) sides with signal drop on apparent diffusion coefficient images along the periarticular surface of right SIJ involving both sacral (red arrow)and iliac (yellow arrow) sides (g). This was scored severe according to Spondyloarthritis Research Consortium of Canada scoring system.
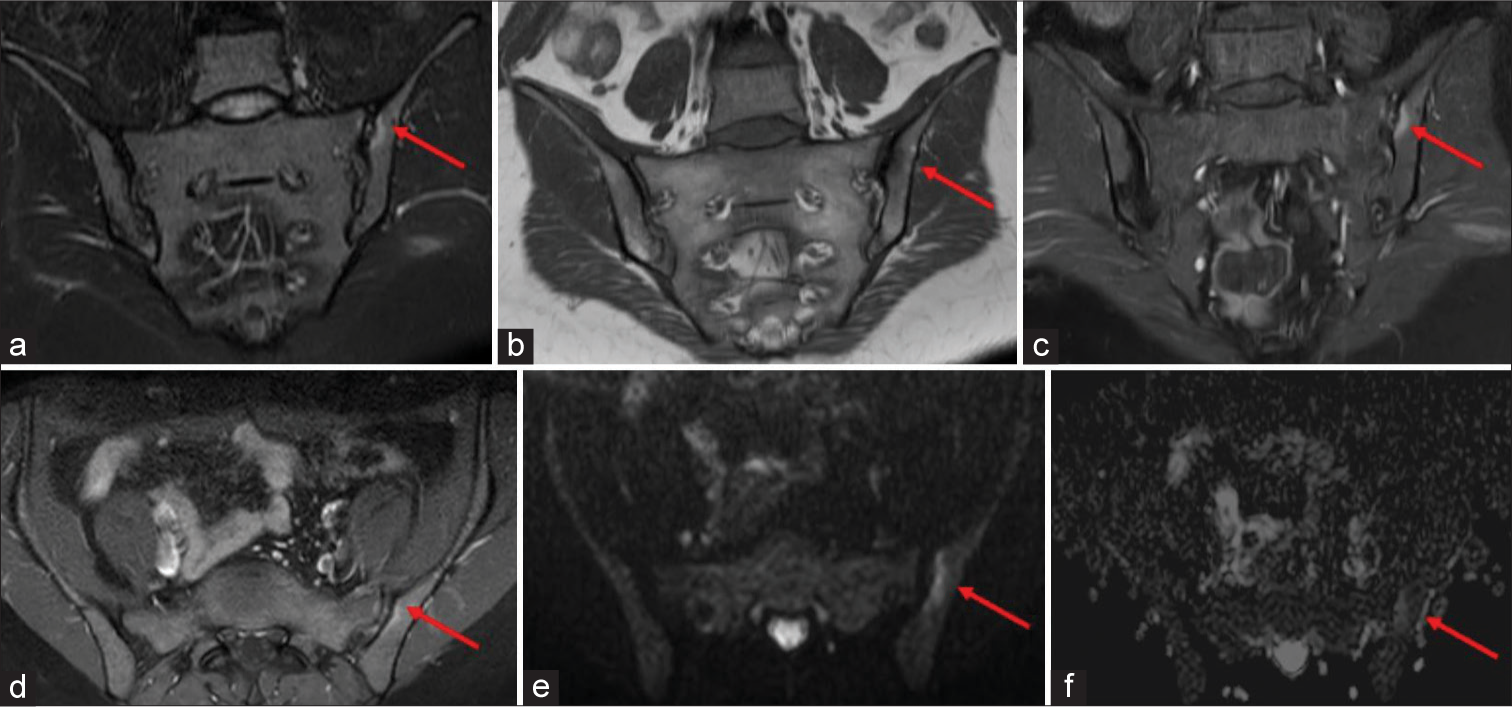
- Magnetic Resonance images of the sacroiliac joints of a 28 years old female patient with negative Human leukocyte antigen LA-B27 with raised C Reactive Protein (12.8 mg/L) and erythrocyte sedimentation rate (36 mm/hr) (a) Oblique coronal Short-tau inversion recovery image showing faint hyperintensity along the iliac periarticular surface of left Sacroiliac joint (red arrow), (b) Oblique T1 image in the coronal plane showing hypointensity as shown by red arrow. (c,d) T1 Weighted fat saturated post contrast images in coronal (c) and axial (d) planes showing enhancement along the periarticular surface of left Sacroiliac joint involving iliac side (red arrows). (e) Oblique diffusion weighted image showing hyperintensity along the iliac periarticular surface of left SIJ showing diffusion restriction along this area with signal drop on apparent diffusion coefficient images in image (f) as shown by red arrows. This was scored moderate according to Spondyloarthritis Research Consortium of Canada scoring system.
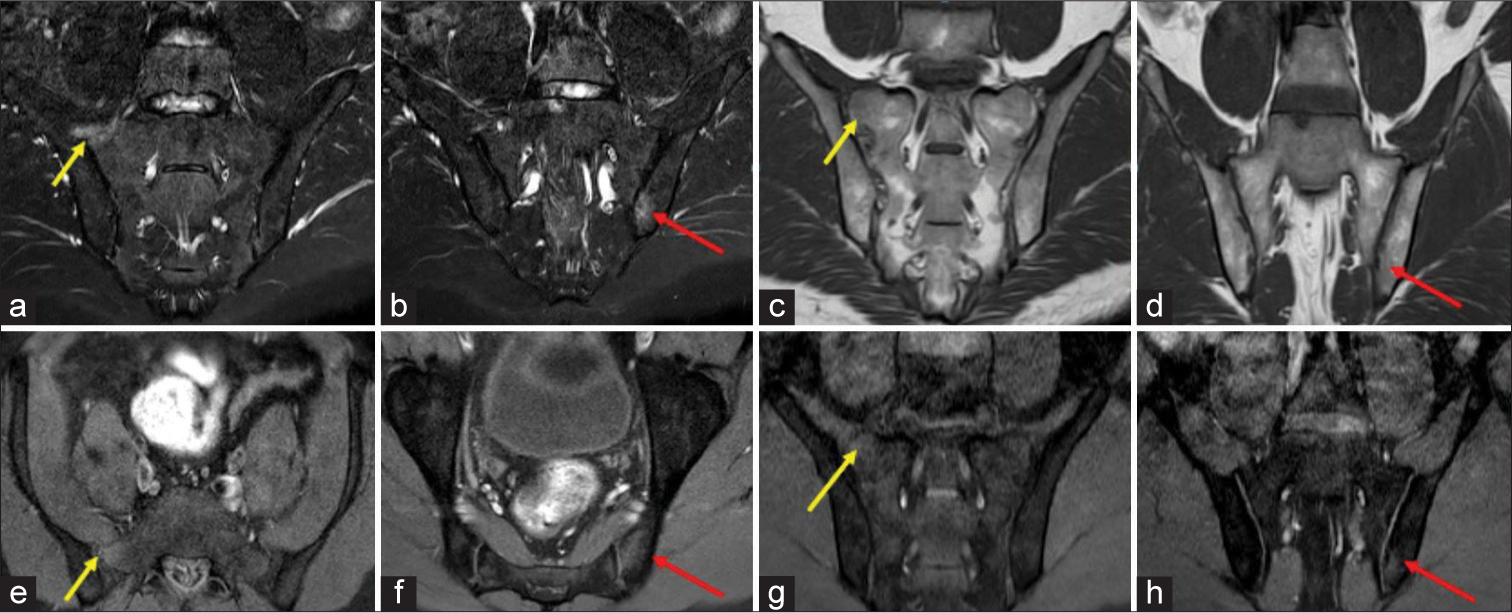
- Magnetic resonance images of the sacroiliac joints of a 31-years-old male patient with negative human leukocyte antigen-B27 with raised C-reactive protein (16.4 mg/L) and erythrocyte sedimentation rate (26 mm/h). (a) Oblique coronal STIR image showing hyperintensity along the periarticular surface of sacral ala in the right sacroiliac joint (SIJ) representing marrow edema as shown by the yellow arrow. (b) Two sections lower from this level show STIR hyperintensity in the left SIJ along the iliac surface as shown by the red arrow. (c and d) Oblique coronal T1 images show faint hypointensities in bilateral SIJ at different levels as shown by yellow arrows (right sacral ala region) and red arrows (left iliac region). (e-h) T1-weighted fat-saturated post-contrast images in axial and coronal planes show enhancement of the above-described areas in the right SIJ (as shown by yellow arrows in e and g) and left SIJ (as shown by red arrows in f and h). This was scored moderate according to the Spondyloarthritis Research Consortium of Canada scoring system.
DISCUSSION
Inflammatory low back pain at the beginning of young adulthood is commonly experienced by patients who have sacroiliitis. Low back pain is one of the most mutual symptoms in most of common people, and surprisingly sacroiliitis is not generally considered in differential diagnosis at first by the clinician. It is difficult to diagnose sacroiliitis at early stages on conventional radiography, and this may lead to a delay of diagnosis by approximately 8 years from the onset of symptoms.[14] In our study, the most common chief complaint was low back ache with some of them presenting with leg radiculopathy, uveitis, and swelling of fingers.
The pattern of involvement can be unilateral or bilateral and ranges in severity from mild-to-severe inflammation resulting in partial or complete ankylosis. The pattern of sacroiliitis in AS is typically bilateral and symmetrical in 85–90% of cases. Other SpA subgroups tend to affect the SI joints in a unilateral or asymmetric manner and are less commonly bilateral, however, most eventually progress to AS.[15,16] In our study, most of the patients presented with bilateral involvement of SIJ with the iliac side involved more common than the sacral side.
Fat-suppressed fluid-sensitive MRI sequences such as STIR and fat-saturated T2-weighted imaging are highly sensitive for the detection of periarticular or subchondral BME, which is central to the diagnosis of active sacroiliitis. Gadolinium contrast-enhanced fat-suppressed T1-weighted sequences are able to improve sensitivity and diagnostic confidence to detect early disease activity but routine contrast administration for all patients is not required.[17,18] Furthermore, in our study contrast and diffusion images showed the same depth of edema consistent with STIR sequences.
Active inflammatory lesion on MRI is known to develop chronic lesion such as fat metaplasia, and fat metaplasia has an increased risk of new bone formation.[19] In our study, there were also findings of fatty marrow changes, bone erosions, ankylosis, and reduced joint space. Various MRI scoring methods exist to quantitatively evaluate the inflammation and BME in patients with sacroiliitis. These scoring systems differ regarding the MRI planes and sequences used to detect inflammation, a more precise quantification for the unit of interest (SI joint divided into halves or quadrants), the number of slices used to score, global versus more extensive grading, and the site of the inflammatory lesion to be scored.[20] Among these, the SPARCC scoring system is the most valuable because of its comprehensive nature and scoring based objectively according to a standardized measurement protocol. While other scoring systems account only for the presence or absence of BME, the SPARCC scoring system incorporates two other MRI indices of potential clinical significance: Signal intensity and the 3-D extent of inflammation.
Our study also included exploratory analysis to determine trends in correlation between the SPARCC scores and clinical variables of disease activity including the inflammatory markers.
A previous study conducted by a French cohort showed that the baseline radiologic lesions in early axial spondyloarthritis (axSpA) were positively associated with CRP level and active inflammation shown in MRI of the SIJ,[7,21] and these results are consistent with the present study as both CRP and ESR were raised in higher grades of inflammatory sacroiliitis. Sacroiliitis grade was the most important factor which could predict the inflammatory SPARCC score of SIJ. Therefore, it could sustenance that axSpA patients with severe baseline sacroiliitis have a greater possibility of acquiring active inflammatory lesions in SIJ.
A previous study from Denmark showed a positive association between HLA-B27 and BME of SIJ.[22] In the present study, HLA-B27 positivity was also associated with increasing inflammatory SPARCC score of SIJ although the number of patients with HLA-B27 positivity was relatively low (30% positive) in the present study. Thus, further studies with larger sample sizes are needed to clarify the association between HLA-B27 and the inflammatory SPARCC score of SIJ.
Some studies have reported the roles of both neutrophils and lymphocytes in pathogenesis. Complete blood count (CBC) and its subtypes are recognized as inflammatory markers in many diseases. Subclinical inflammatory parameters such as neutrophil to lymphocyte ratio have been found to be associated with inflammation in diseases such as Familial Mediterranean Fever. However, the relationships between these subclinical inflammatory parameters and disease activity in axSpA have not been fully understood yet. In some studies, neutrophilto-lymphocyte ratio and platelet-to-lymphocyte ratio were reported to be significantly higher in AS patients with severe disease activity compared to mild disease activity.[23] In our study, CRP (P < 0.001 and r = 0.62) and ESR (P < 0.001 and r = 0.85) were found to be significantly higher in patients with higher grades of sacroiliitis, congruent with previous studies.
CRP and ESR are the most widely used laboratory indices for the estimation of disease activity. In recent years, acute-phase reactants have been playing a prominent role in monitoring patients with axSpA. In this regard, like acute-phase reactants, subclinical inflammation indices obtained from CBC and particularly neutrophil-to-lymphocyte ratio may have contributed to disease activity assessment. However, this needs to be confirmed in further studies.[24]
In a study conducted, elevated CRP and alternating buttock pain were significantly more common in AS group, and the level of inflammatory biochemical parameters and total inflammatory SPARCC score of SIJ were significantly higher in AS group than in the non-radiographic axial spondyloarthritis (nrAxSpA) group. The results for spondyloarthritis which was diagnosed earlier, demonstrated that the inflammatory lesions of the SIJ showed a significant association with buttock pain,[25] and this might support the higher frequency of alternating buttock pain and higher inflammatory SPARCC score of SIJ in the AS group. The aforementioned results support that AS patients are more frequently in the acute inflammatory state than nrAxSpA in an aspect of biochemical parameters and MRI findings.[26]
In addition to inflammatory sacroiliitis, various other SIJ abnormalities on MRI in patients without axSpa do exist. The differential diagnosis includes osteoarthritis, osteitis condensans ilii, postpartum changes, dysmorphic SIJ, accessory SI joint, insufficiency fractures, infections, etc. These can be differentiated based on the pattern of involvement, clinical history, and laboratory findings.[27]
There were few limitations to the study. The data collected was from a single tertiary hospital and the grades of sacroiliitis were based on MRI findings. Secondly, the study was cross sectional and included only baseline data.
CONCLUSION
In conclusion, inflammatory sacroiliitis presents with chief complaints of low-backache. MRI findings help us to grade the early disease into mild, moderate and severe degree. STIR is the most sensitive sequence for detection of bone marrow oedema with symmetrical involvement of both sides, however, the iliac bone is more commonly involved than the sacral aspect of the SIJ. Contrast enhanced images and diffusion sequences offer no additional advantage in the diagnosis of BME in inflammatory sacroiliitis. HLA-B27, although not specific to inflammatory sacroiliitis, is increased in higher grades of sacroiliitis.
Acknowledgments
I would like to acknowledge Mr. Sanjeev Kumar Bhatia, Mr. Bhajan Singh Bhullar (MRI Technicians), Mr. Rohit Sharma, and Mr. Sarbjit Singh; department of Radiodiagnosis for their support.
Ethical approval
The research/study is approved by the Institutional Ethics Committee at SGRDIMSR, number Ant/94/2021, dated 19th February 2021.
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- MRI of the sacroiliac joints: What is and what is not sacroiliitis? Curr Opin Rheumatol. 2020;32:357-64.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis-cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135-40.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of MRI features of sacroiliitis in juvenile spondyloarthritis. Clin Radiol. 2015;70:1428-38.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variation of the sacroiliac joints: An MRI study with synthetic CT images. Insights Imaging. 2023;14:30.
- [CrossRef] [PubMed] [Google Scholar]
- Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: Update by the ASAS MRI working group. Ann Rheum Dis. 2016;75:1958-63.
- [CrossRef] [PubMed] [Google Scholar]
- Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:703-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of diffusion-weighted MRI in the detection of early active sacroiliitis. AJR Am J Roentgenol. 2008;191:980-6.
- [CrossRef] [PubMed] [Google Scholar]
- MRI in seronegative spondyloarthritis: Imaging features and differential diagnosis in the spine and sacroiliac joints. AJR Am J Roentgenol. 2013;200:149-57.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging of active sacroiliitis: Do we really need gadolinium? Eur J Radiol. 2009;71:232-6.
- [CrossRef] [PubMed] [Google Scholar]
- HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: Results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis. 2011;70:1930-6.
- [CrossRef] [PubMed] [Google Scholar]
- Demographic, clinical, laboratory and treatment characteristics of spondyloarthritis patients with and without acute anterior uveitis. Sao Paulo Med J. 2012;130:141-4.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of magnetic resonance image and HLA-B27 in early detection of sacroiliitis in Egyptian spondyloarthropathic patients. Egypt Rheumatol Rehabil. 2015;42:137-44.
- [CrossRef] [Google Scholar]
- Relationship of HS CRP and sacroiliac joint inflammation in undifferentiated spondyloarthritis. Open Med (Wars). 2018;13:113-8.
- [CrossRef] [PubMed] [Google Scholar]
- C-reactive protein, ESR, and Klebsiella in ankylosing spondylitis. Ann Rheum Dis. 1980;39:45-9.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between magnetic resonance imaging (MRI) findings and the new bone formation factor Dkk-1 in patients with spondyloarthritis. Clin Rheumatol. 2019;38:465-75.
- [CrossRef] [PubMed] [Google Scholar]
- The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25-31.
- [CrossRef] [PubMed] [Google Scholar]
- ASDAS is associated with both the extent and intensity of DWMRI spinal inflammation in active axial spondyloarthritis. RMD Open. 2019;5:e001008.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between diagnostic imaging findings of sacroiliitis and inflammation parameters. Akt Rheumatol. 2022;47:61-8.
- [CrossRef] [Google Scholar]
- Association of genetic marker HLA-B27 with spondyloarthritis in a tertiary care centre in South India. J Pure Appl Microbiol. 2022;16:901-8.
- [CrossRef] [Google Scholar]
- Neutrophil-lymphocyte ratio connected to treatment options and inflammation markers of ankylosing spondylitis. J Clin Lab Anal. 2015;29:294-8.
- [CrossRef] [PubMed] [Google Scholar]
- Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 2012;64:1388-98.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between spondyloarthritis features and magnetic resonance imaging findings: A cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol. 2016;68:892-900.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the assessment of spondyloarthritis international society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 2018;70:1042-8.
- [CrossRef] [PubMed] [Google Scholar]
- Is the site of back pain related to the location of magnetic resonance imaging lesions in patients with chronic back pain? Results from the spondyloarthritis caught early cohort. Arthritis Care Res (Hoboken). 2017;69:717-23.
- [CrossRef] [PubMed] [Google Scholar]
- Main diagnostic pitfalls in reading the sacroiliac joints on MRI. Diagnostics (Basel). 2021;11:2001.
- [CrossRef] [PubMed] [Google Scholar]







