Translate this page into:
Dorsal Epidural Artery Pseudoaneurysm – An Unusual Complication after Posterior Instrumented Stabilisation

*Corresponding author: Alagiri Karikalan, Department of Radiology, Ganga Medical Centre and Hospital, Coimbatore - 641 009, Tamil Nadu, India. drazhagiri@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Karikalan A, Pushpa BT, Ramesh S, Kuna VS. Dorsal Epidural Artery Pseudoaneurysm – An Unusual Complication after Posterior Instrumented Stabilisation. Indian J Musculoskelet Radiol 2020;2(2):156-60.
Abstract
Vascular complications during lumbar disc surgeries are a rare but fatal complication. Most commonly injured vessels during spinal surgeries include the aorta, common iliac vessels, and very rarely the lumbar arteries. The damage to a major vessel such as aorta and iliac artery present with acute symptoms requiring immediate surgical intervention, whereas small vessels such as lumbar segmental artery and its branches when injured may form arteriovenous fistula and pseudoaneurysm presenting at a later stage. We report a rare case of dorsal epidural artery pseudoaneurysm following lumbar discectomy and posterior instrumented stabilization presenting with progressive signs of neurological deficit due to dural sac compression and also emphasize the importance of its early diagnosis thus preventing fatal complications.
Keywords
Pseudoaneurysm
Epidural artery
Lumbar artery
Spine surgery complication
INTRODUCTION
Lumbar disc surgery is one of the most commonly performed neurosurgical procedure related to the spine. Vascular complications related to posterior lumbar disc surgery are not so common.[1] The radiologist should be aware of late vascular complications in patients with progressive neurological symptoms following spinal surgery. Early diagnosis and prompt treatment are essential and thus can prevent fatal outcomes of this condition. Treatment may be either surgical or endovascular repair based on the type of injury. In this article, we report a rare case of pseudoaneurysm of the dorsal epidural artery causing spinal canal compression, presenting with neurological deficit in the post-operative period.
CASE REPORT
A sixty years old female presented with complaints of low backache with left lower limb radiculopathy for the past 6 years and difficulty in walking for the past 2 months. The pain was insidious in onset with a gradual increase in severity. There was no bowel and bladder involvement. She was under antiplatelet medications for ischemic heart disease. Local neurological examination of both lower limbs was within normal limits except for decreased sensation in the left L4, L5, and S1 dermatome. Her imaging workup showed L3-4 and L4-5 Grade I degenerative spondylolisthesis on plain radiograph. Magnetic resonance imaging (MRI) revealed disc bulge impinging on the L3-4 and L4-5 exiting nerve roots [Figure 1]. She was planned for L3-5 decompression and interbody fusion. Intraoperatively midline decompression was achieved by L3, L4, and L5 laminectomy, bilateral pedicle screws were fixed at L3, L4, and L5 vertebra and L3-4 posterolateral fusion was done using an autologous bone graft. The intraoperative and immediate post-operative period was uneventful, so the patient was discharged after 1 week.
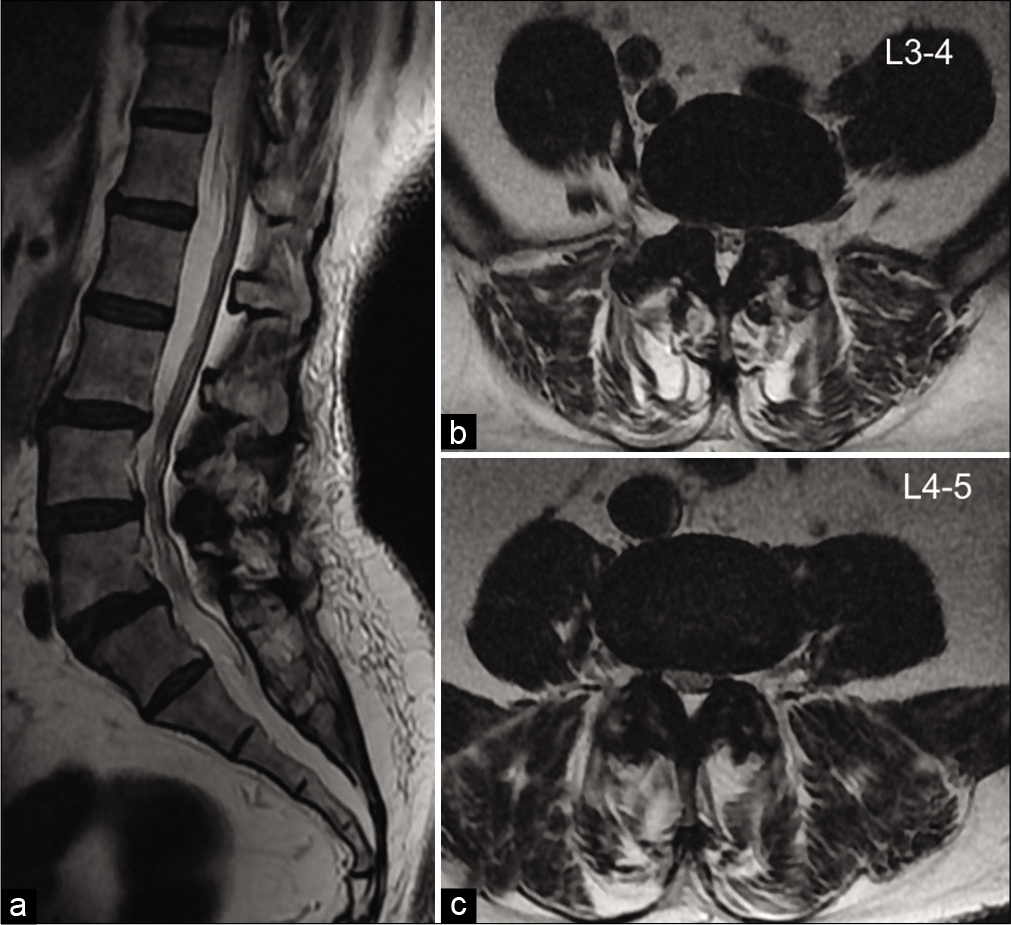
- Sagittal and axial T2-weighted MR images showing; (a) Grade I anterolisthesis of L3 over L4 and L4 over L5 (b and c). Circumferential disc bulge is causing moderate spinal canal and left neural foramen narrowing impinging on the left exiting nerve roots at L3-4 and L4-5 levels.
One week later, she presented to the emergency room with complaints of severe left hip and lower limb pain, inability to stand, and inability to micturate since afternoon. The pain was gradually progressing and associated with paraesthesia up to the left foot. As the patient was stable, MRI of the lumbar spine was done, which revealed a large organized collection in the paraspinal region apart from the post-operative changes. This collection was isointense on T1-weighted, heterogeneously hyperintense on T2-weighted, and TRIM sequences suggesting a hyperacute to acute hematoma. The collection was extending into the epidural space causing significant compression of the cauda equina nerve roots and also mass effect over the adjacent paraspinal muscles [Figure 2]. However, the information was inadequate due to magnetic susceptibility artefacts. Hence, we decided to do an ultrasound screening which showed a heteroechoic collection with mobile internal echoes and a relatively well defined anechoic pulsatile lesion within. On colour Doppler study, the lesion demonstrated the classical “Yin-Yang sign” and arterial spectral waveform suggesting an arterial aneurysm [Figure 3]. To precisely locate the site of aneurysm contrast computed tomography (CECT) was done.
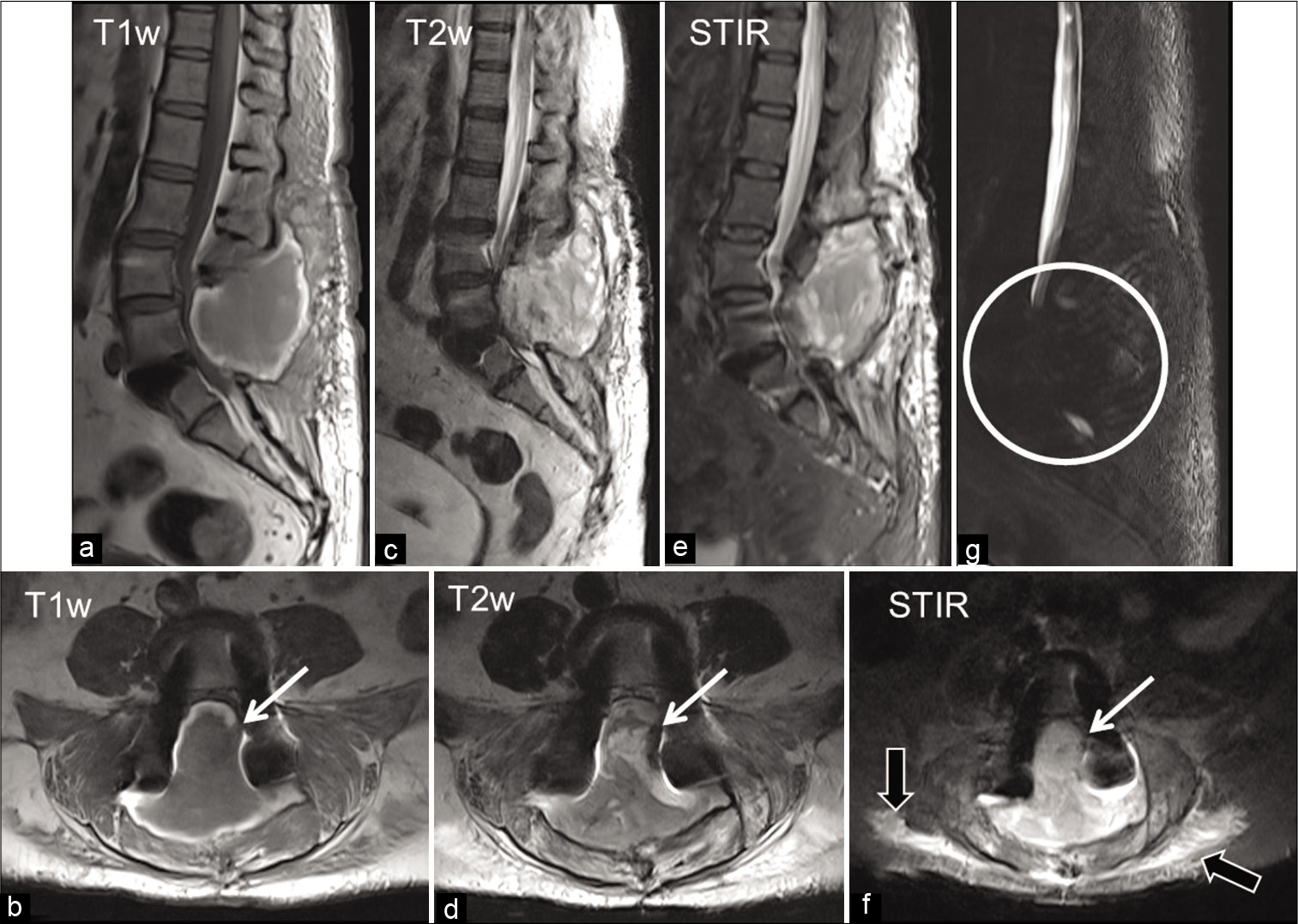
- (a-f) Sagittal and axial MR images of the lumbar spine showing: A large paraspinal collection isointense on T1-weighted image (WI) with faint areas of hyperintensity within and peripheral hyperintense rim (a and b), heterogeneously hyperintense on T2WI (c and d), and short-TI inversion recovery (e and f) images. The collection is seen clearly extending into the epidural space (white arrow) causing significant compression of the cauda equina nerve roots and also mass effect over the adjacent paraspinal muscles (black arrows). (g), MR myelography showing complete obliteration of the cerebrospinal fluid space at that level (circle). Bilateral pedicle screws are causing magnetic susceptibility artefacts.
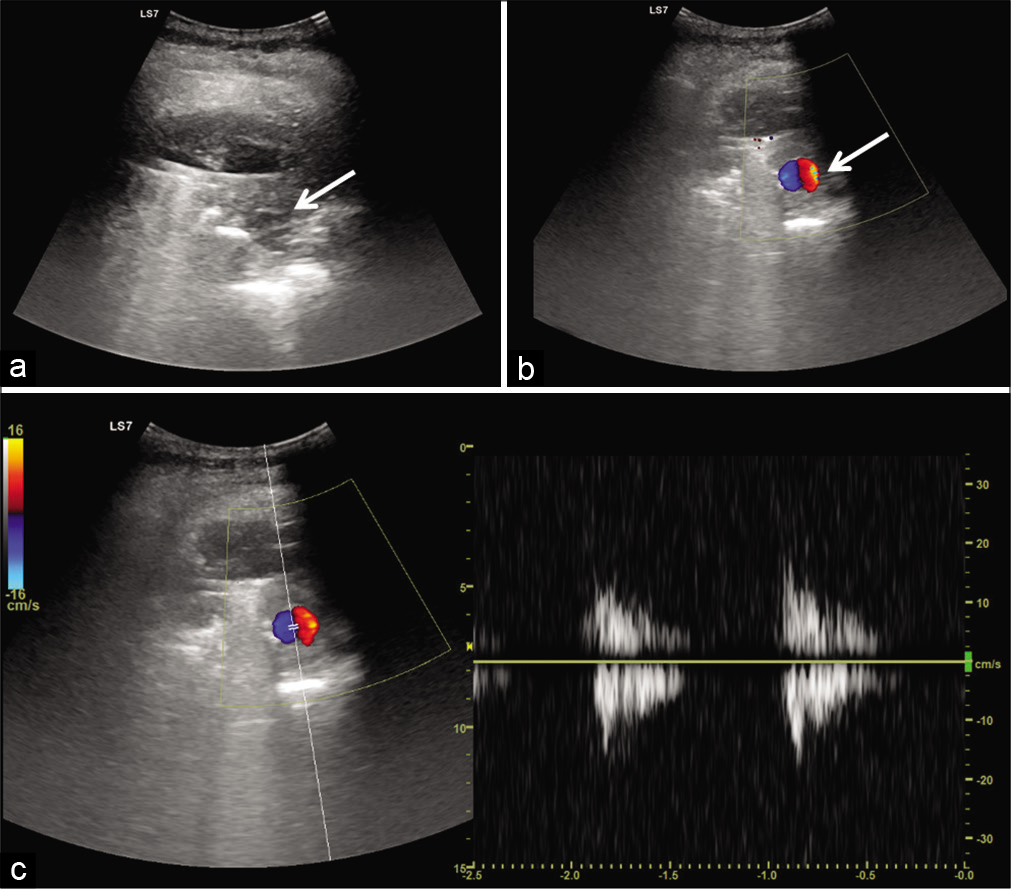
- Ultrasonogram of the collection site shows (a) heteroechoic collection with internal echoes and a relatively well defined anechoic lesion (arrow) (b) color Doppler showing a typical “Yin-Yang sign” (white arrow) and arterial waveform (c) suggesting an arterial aneurysm.
The CECT showed a well-defined round, homogeneously enhancing lesion in the left posterior paramedian epidural space adjacent to the left L4 pedicle screw. The lesion was faintly enhancing in the arterial phase, filling up in the venous phase, and washout on delayed phase [Figure 4]. No obvious feeding or draining vessel were detected. These findings lead to the conclusion of a pseudoaneurysm arising from a branch of the left L4 segmental artery. Following this, the patient was referred for endovascular embolization, the procedure revealed the exact source of bleeding to be left dorsal epidural artery at L4 level. Post-embolization the patient’s symptoms gradually subsided and she became ambulatory.
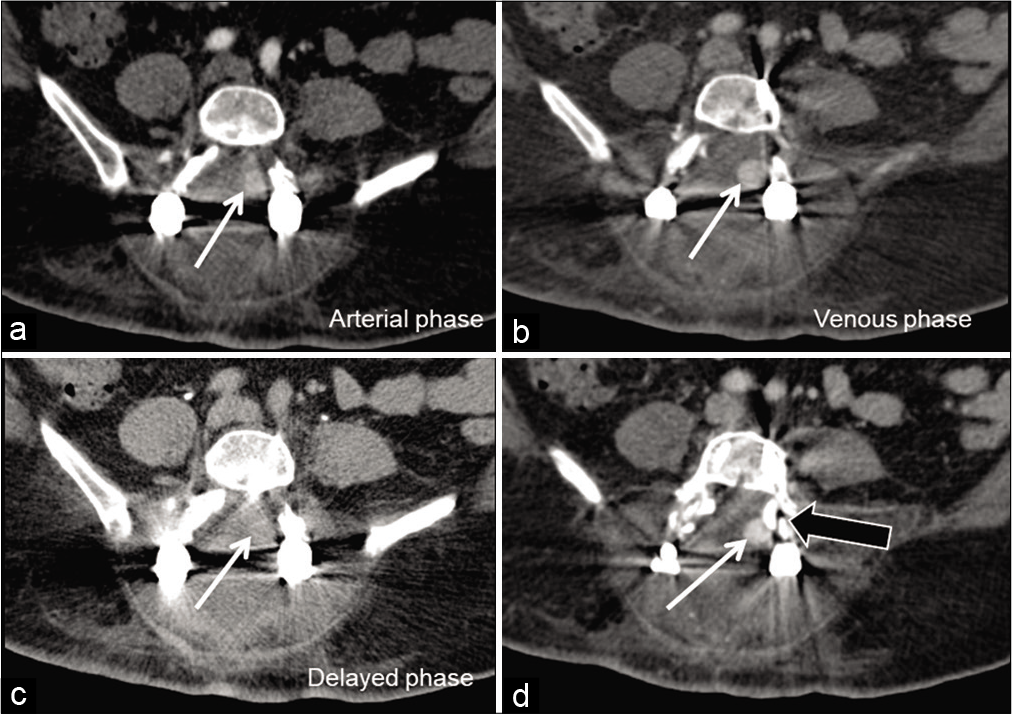
- Contrast-enhanced axial computed tomography showing a well-defined round, homogeneously enhancing lesion (white arrows) in the left posterior paramedian epidural space adjacent to the left L4 pedicle screw (black arrow). The lesion showed faint enhancement in arterial phase (a), filling up and more evident in the venous phase (b), and washout on delayed phase (c) suggesting the possible origin of pseudoaneurysm from left branch of L4 lumbar artery.
DISCUSSION
Iatrogenic vascular injury during intervertebral disc surgery is a well-recognized but uncommon complication. However, the incidence of lumbar artery injury is rare and is documented only in a limited number of case reports.[1] The reported incidence of vascular complications of spinal surgery ranges from 0.01% to 0.22% and the mortality rate has been reported to range from 15% to 65%. The aorta and the iliac vessels are at increased risk of injury due to their proximity to the vertebral column.[2,3] Bingol et al. have also stated that the most commonly injured vessels in their study were left common iliac artery and left common iliac vein.[4]
The vascular complications resulting from spinal surgeries can be broadly divided into early and late-onset complications. Early-onset complications occur either intraoperatively or a few hours after the surgery.[2,3] Late-onset complications due to vascular injury may manifest from a few days to years after the surgical procedure. The most common late complications are arteriovenous fistula (AVF) and pseudoaneurysm.[5] Kwai-Tung Chan and Naveen Korivi reported that the clinical presentation of lumbar artery pseudoaneurysm can be delayed. The signs and symptoms of lumbar artery injury are less appreciated in the immediate post-operative period due to the poorly localized and non-specific symptoms.[6] This results in a delay in diagnosis, subsequently leading to the development of AVF or pseudoaneurysm. Iatrogenic lumbar artery pseudoaneurysm formation has been described after discectomy and posterior lumbar realignment surgeries.[1,4,7]
The principal arterial supply to the spine is derived from the segmental arteries and their anastomotic plexus. The segmental arteries for the upper four lumbar vertebrae arise from the descending abdominal aorta. The L5 vertebra and sacrum derive their blood supply from the internal iliac and median sacral arteries. The segmental arteries course laterally along the surface of the vertebral body and divide into three main branches namely ventral (lumbar) artery, dorsal (muscular and cutaneous), and medial (spinal) [Figure 5]. The spinal branch at each segment enters the spinal canal through the neural foramen and divides into (a) dorsal and ventral spinal canal (epidural) arteries. These arteries supply the posterior vertebral elements, ligaments and to a lesser extent dura mater, and (b) a radicular branch that supplies the dura mater and nerve root at corresponding levels.[8]
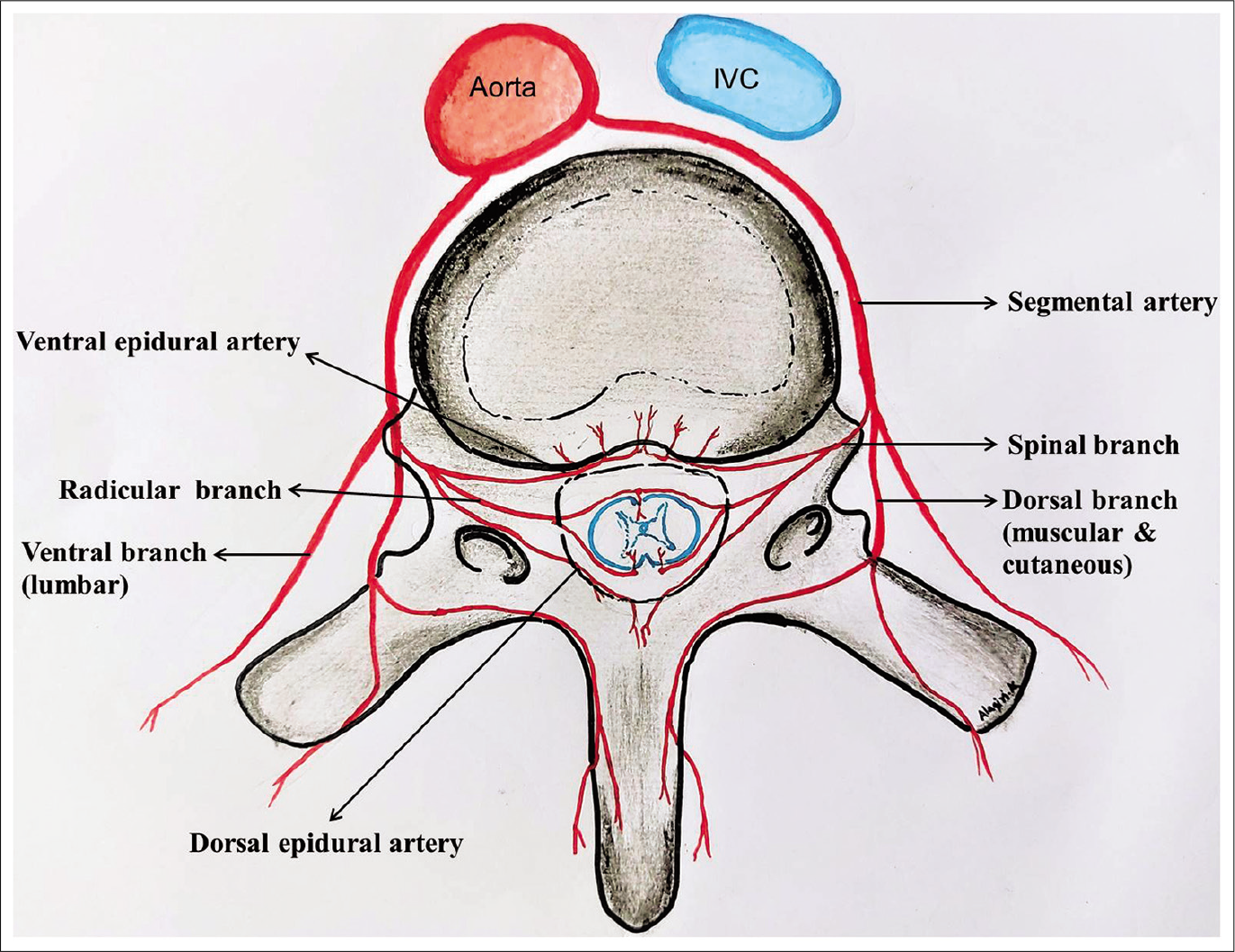
- Schematic representation of blood supply of the spinal column and spinal cord.
A pseudoaneurysm is caused by damage to the arterial wall, resulting in locally contained hematoma with turbulent blood flow and a neck that communicates with the arterial lumen. Bleeding from the lumbar arteries is initially contained by the surrounding retroperitoneal soft tissues, which act as a tamponade forming a pseudoaneurysm.[6]
Lumbar artery pseudoaneurysm may be asymptomatic or can present with non-specific signs and symptoms. Pain is the most common presenting symptom which may result from the stretching of surrounding nerves or rupture of the pseudoaneurysm.[6] The time between injury and onset of symptoms is variable, with delays of up to 18 months having been reported.[9] In our case, the left paramidline location of the pseudoaneurysm occurred close to the medial border of the left pedicle suggesting injury to a small branch of lumbar segmental artery which was later identified as left dorsal epidural artery in angiography during embolization. Laminectomy at L3, L4, and L5 provides space for the hematoma to expand. The midline location of the expanding hematoma resulted in compression of the dural sac and cauda equina, leading to progressive neurological deficits. Robbert et al. stated that in an intact spinal canal, a lumbar artery pseudoaneurysm may cause early retroperitoneal haemorrhage or chronic compression of a single nerve root, but no compression of the dural sac.[10]
Dorsal epidural artery pseudoaneurysm should be recognized and treated as early as possible due to its tendency to bleed and expand causing neurological symptoms. Our experience in this case, emphasizes that in case of suspected pseudoaneurysm or AVF the combined use of MRI and ultrasound screening of the post-operative site can reveal the possible diagnosis shortening the delay in treatment and thus preventing undue complications.
CONCLUSION
Despite its rare incidence, dorsal epidural artery pseudoaneurysm related to lumbar disc and posterior instrumented stabilization surgery should always be kept in mind in case of worsening symptoms in the postoperative period. The radiologist must be familiar with vascular complications of spinal surgery and should use the appropriate imaging modality for early diagnosis, thus aiding in a timely intervention. Early diagnosis and prompt treatment are essential to prevent fatal outcomes in these patients.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir (Wien). 2006;148:375-87.
- [CrossRef] [PubMed] [Google Scholar]
- Management of major vascular injury during pedicle screw instrumentation of thoracolumbar spine. Clin Neurol Neurosurg. 2017;163:53-9.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and clinical significance of vascular encroachment resulting from freehand placement of pedicle screws in the thoracic and lumbar spine: Analysis of 6816 consecutive screws. Spine (Phila Pa 1976). 2014;39:683-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular complications related to lumbar disc surgery. J Neurosurg. 2004;100(Suppl 3):249-53.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular complications of lumbar disc surgery. Case report. Eur J Surg. 1991;157:141-3.
- [Google Scholar]
- Lumbar artery pseudoaneurysm in traumatic spinal cord injury: A case report. Arch Phys Med Rehabil. 2003;84:455-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular injury complicating lumbar disc surgery. A systematic review. Eur J Vasc Endovasc Surg. 2002;24:189-95.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular anatomy of the spinal cord. J Neurointerv Surg. 2012;4:67-74.
- [CrossRef] [PubMed] [Google Scholar]
- Lumbar artery pseudoaneurysm: CT demonstration. J Comput Assist Tomogr. 1984;8:570-2.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic lumbar pseudoaneurysm causing dural sac compression after spine surgery. J Neurosurg Spine. 2009;10:585-6.
- [CrossRef] [PubMed] [Google Scholar]






