Translate this page into:
Giant glomus tumor of the knee mimicking soft-tissue sarcoma

*Corresponding author: Vikas Batra, Department of Radiology, Mahajan Imaging, Sports Injury Center, Safdarjung Hospital, Ansari Nagar, New Delhi - 110 029, India. drvikas28@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Batra V, Batta NS, Gupta A. Giant glomus tumor of the knee mimicking soft-tissue sarcoma. Indian J Musculoskelet Radiol 2020;2(1):82-6.
Abstract
Glomangiomas (glomus tumors) are benign vascular tumors commonly located at the distal extremities, are usually subungual lesions, and account for 2% of all soft-tissue tumors. Patients with digital glomus tumors present with hypersensitivity to cold, paroxysmal severe pain, and point tenderness. These tumors are infrequent in the knee area, and when seen are superficial, usually have a diameter of less than 1 cm, which make their radiological diagnosis arduous. We report a noteworthy, unusual case of a large glomus tumor in the popliteal fossa showing biceps femoris infiltration, in a 51-year-old female patient who experienced severe intermittent posterior knee pain for the past 2 years. Magnetic resonance imaging revealed a large popliteal inhomogeneous soft-tissue lesion with irregular margins insinuating the posterolateral musculature mimicking soft-tissue sarcoma. Histopathology revealed a glomus tumor.
Keywords
Glomus tumor
Soft-tissue sarcoma
Glomangioma
Knee
INTRODUCTION
Glomus tumor is usually a benign neoplasm. It is a perivascular mesenchymal tumor arising from the glomus body which is a thermoregulatory apparatus within the dermis. It is most commonly seen in subungual region of the finger but may occur anywhere. It is one of the characteristic fingertip masses that can be diagnosed with magnetic resonance imaging (MRI). These lesions occur in adults aged 20–40 years with subungual lesions showing female predominance. These tumors are infrequent in the knee area, and when seen are superficial, usually subcentimetric.
CASE REPORT
A 51-year-old female patient presented in our orthopedic outpatient department with severe intermittent pain and fullness in the right popliteal fossa for the past 2 years. It was associated with restricted and painful knee movements. There was no history of trauma or fever. Physical examination revealed swelling in the right popliteal fossa and lower thigh with a decreased range of motion and pain during extension. No neurological deficit was noted. X-ray knee (anteroposterior and lateral) was done, which was inconclusive; hence, magnetic resonance imaging (MRI) was recommended. MRI (1.5 Tesla GE Healthcare Signa HDXT) was performed with axial, coronal, and sagittal images using T1-weighted (W) turbo spin-echo, T2-W turbo spin-echo sequences integrated with fat suppression, and diffusion-weighted imaging (DWI). Contrast imaging was advised, but was refused by the patient.
MRI non-contrast study showed a large heterogeneous ill-marginated multilobulated lesion in the popliteal fossa and lower thigh, centered in the lateral intermuscular plane, measuring approximately 40 mm anteroposteriorly, 39.9 mm mediolaterally, and 42.6 mm in craniocaudal dimensions. The lesion was inhomogeneous, showed focal infiltrative margins insinuating into the biceps femoris muscle and in close vicinity to the posterior cortex of the femur without any evidence of cortical erosion/cortical thickening/periosteal reaction or intramedullary invasion. There was no evidence of intralesional hemorrhage or necrotic changes. Overlying skin and subcutaneous tissue were unremarkable. Intimate association to the common peroneal nerve and medial displacement of the popliteal vein/artery was noted.
The lesion demonstrated iso to slightly hyperintense signals on T2W sequence [Video file 1 (please visit the article in HTML to watch the video at https://dx.doi.org/10.25259/IJMSR_9_2020) and Figure 1] and isointense signals to the muscle on T1W sequences [Figure 2], was hyperintense on diffusion-weighted sequence (b value – 1000 s/mm2) and hypointense on apparent diffusion coefficient sequence [Figure 3]. Hence, the preliminary diagnosis of soft-tissue sarcoma was made based on insinuation into the biceps margins [Figure 4].
![Axial T2-weighted (a) and sagittal proton density-weighted (b) images reveal iso to slightly hyperintense (relative to muscle) lesion (asterisk) with irregular spiculated margins abutting the posterior cortex of femur without any cortical erosion and showing contiguous infiltration into the adjoining distal biceps femoris muscle. The lesion is causing splaying of the neurovascular bundle (arrow) (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, TE for T2-weighted image [T2WI] – 91, TR for T2WI 4580, TE for platelet distribution width [PDW] – 19.7, TR for PDW – 2000).](/content/107/2020/2/1/img/IJMSR-2-082-g001.png)
- Axial T2-weighted (a) and sagittal proton density-weighted (b) images reveal iso to slightly hyperintense (relative to muscle) lesion (asterisk) with irregular spiculated margins abutting the posterior cortex of femur without any cortical erosion and showing contiguous infiltration into the adjoining distal biceps femoris muscle. The lesion is causing splaying of the neurovascular bundle (arrow) (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, TE for T2-weighted image [T2WI] – 91, TR for T2WI 4580, TE for platelet distribution width [PDW] – 19.7, TR for PDW – 2000).
![A 51-year-old woman who presented with severe pain in the right popliteal fossa for the past 2 years. Axial T1-weighted image reveals iso-intense (to muscle) lesion (asterisk) in the posterior aspect of the lower thigh and popliteal fossa with irregular spiculated margins abutting the posterior cortex of femur (arrow) without any cortical erosion (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, TE – 8.4, TR – 820).](/content/107/2020/2/1/img/IJMSR-2-082-g002.png)
- A 51-year-old woman who presented with severe pain in the right popliteal fossa for the past 2 years. Axial T1-weighted image reveals iso-intense (to muscle) lesion (asterisk) in the posterior aspect of the lower thigh and popliteal fossa with irregular spiculated margins abutting the posterior cortex of femur (arrow) without any cortical erosion (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, TE – 8.4, TR – 820).
![On axial diffusion weighted (a) (at b value 1000 s/mm2) and apparent diffusion coefficient (b) sequences: Lesion (asterisk) reveals internal hyperintense (a) and hypointense (b) signals, respectively, representing diffusion restriction and high cellularity (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, b value for diffusion-weighted imaging (DWI) – 1000, TE for DWI – 82.9, TR for DWI – 3600).](/content/107/2020/2/1/img/IJMSR-2-082-g003.png)
- On axial diffusion weighted (a) (at b value 1000 s/mm2) and apparent diffusion coefficient (b) sequences: Lesion (asterisk) reveals internal hyperintense (a) and hypointense (b) signals, respectively, representing diffusion restriction and high cellularity (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, 1.5 mm interslice gap, b value for diffusion-weighted imaging (DWI) – 1000, TE for DWI – 82.9, TR for DWI – 3600).
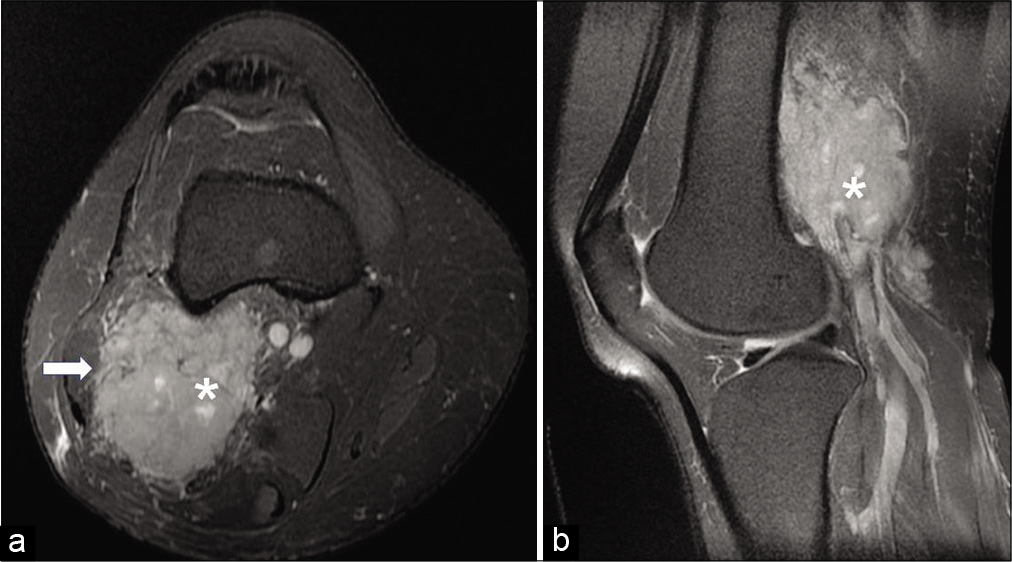
- On axial (a) and sagittal proton density (b) fat-saturated images, the lesion (asterisk) appears hyperintense and insinuating into the biceps femoris muscle (arrow) (acquisition protocol – 1.5 Tesla scanner (GE Signa HDxt), 4 mm slice thickness, interslice gap for axial PDFS 1.5 mm, interslice gap for sagittal PDFS 1.0 mm, TE for axial PDFS 51.1, TE for sagittal PDFS 43.1, TR for axial PDFS 3080, TR for sagittal PDFS 2480).
The patient underwent excision biopsy, and the tumor margins were negative for tumor cells. Histopathology revealed tumor comprising lobules of varying sizes separated by thick fibrous septa, with large intranuclear inclusions in few cells, no necrosis seen. The mitotic index was negligible. The tumor cells expressed smooth muscle actin and integrase interactor 1 and are immunonegative for desmin, h-caldesmon, HMB45, melan A, CD 34, cytokeratin, and EMA. Histopathology and immunochemistry findings were consistent with glomus tumor.
DISCUSSION
Glomangiomas (glomus tumors) are benign vascular tumors predominantly seen in distal extremities. Patients typically present with paroxysmal severe pain, cold intolerance, and exquisite tenderness to touch.[1,2] These tumors are usually subungual lesions and account for 2% of all soft-tissue tumors.[2] Extradigital glomus tumors are rarely seen and thus difficult to diagnose.
These tumors are very infrequent in the knee area, and when seen, usually have a diameter of less than 1–2 cm, which makes their diagnosis arduous and disadvantageous to treatment.[3] Furthermore, cases of extradigital glomus tumors are often misinterpreted, due to their small size and unusual location.
Therefore, we report a noteworthy, strikingly unusual case of a large glomus tumor in the popliteal fossa extending into the lower thigh of a 51-year-old female patient who experienced severe posterior knee pain for the past 2 years.
Glomus bodies are perivascular temperature regulating structure located at the tip of a digit or beneath the nail functioning as arteriovenous shunts. The glomus tumor is actually a mesenchymal hamartoma arising from the glomus body.[1,2] Lesions are predominantly observed in females in the third and fourth decades characteristically in the fingertip as subungual lesions.[1,2] Patients generally present with pinpoint pain in the subungual region. On physical examination, a small bluish subungual nodule can be seen with nail ridging or discoloration. Some of the patients present with the classical triad of cold sensitivity, severe intermittent pain, and pinpoint localized tenderness.[1,2] The Hildreth’s and Love’s pin tests are reliable sensitive and specific methods of diagnosing glomus tumors. MRI is used to diagnose this characteristic fingertip mass with a high level of precision.
In 1924, Masson described histologic variants of glomus tumors.[4] Depending on the histologic composition, variations in the MR signal characteristics are noted. Three types of variants are generally seen.
Vascular type
It is comprising vascular lumen, hence exhibiting hyperintensity on T2, and showing avid early arterial enhancement increasing on the delayed venous acquisition.
Cellular or solid-type
It is comprising epithelioid cells with a paucity of vascular lumens, hence exhibiting T2 iso- to hypointense signals with mild post-contrast enhancement.
Mucoid type
It is characterized by stromal mucoid degeneration, demonstrating T2 hyperintense signals and mild enhancement after gadolinium administration.
As the most common location of occurrence is subungual region at the tip of fingers,[1-3] extradigital glomus tumors can be infrequently seen in other sites such as head and neck, pulmonary, mediastinum, gastrointestinal tract, and within the bones.[1] Extradigital glomus tumors appear to be more common in males as compared to the tumors in the hand, which show a 3:2 female predominance.[2]
Glomus tumors surrounding the knee are rare and can be seen in anterior subcutaneous plane, within the Hoffa’s fat pad and popliteal fossa.[3,5]
Classically, extradigital glomus tumors are seen in a subcutaneous plane around the knee joint showing similar imaging features that are considered diagnostic for typical glomus tumor, including intermediate or low signal intensity on T1-W images and marked hyperintensity on T2-W images. These tumors usually show avid and uniform enhancement on post-contrast sequences.
Multi-voxel proton MR spectroscopy may help in differentiating benign and malignant musculoskeletal tumors. Patni et al.[6] detected a choline peak in all 32 patients with musculoskeletal tumors. They obtained a cutoff choline/ Cr ratio of 12.5 for differentiating malignant from benign musculoskeletal tumors with a sensitivity of 85%, specificity of 83.3%, and diagnostic accuracy of 84.3%.
The literature depicting the extra digital glomus tumor within and around the knee is relatively sparse. Frumuseanu et al.[7] published a case report on subcutaneous glomus tumor in a 10-year-old male patient in the anteromedial aspect of knee. Wong et al.[8] published a case report on glomus tumor in the suprapatellar fat pad of the knee in a 51-year-old male who experienced chronic pain.
To the best of the authors knowledge, only five cases of popliteal fossa glomus tumors have been published in the English language medical literature. Four out of these five cases were subcutaneous/superficial in location [Table 1]. Lekehal et al.[9] published a case report – glomangioma of the popliteal fossa in French medical literature in 1997.
| Patient no. | Age/sex | Size/appearance | Histological finding | Authors |
|---|---|---|---|---|
| 1. | 54/M | Small nodule in adipose tissue | Glomus tumor | Mackenney RP, Reed L. Atypical glomus tumors. J R Coll Surg Edinb. 1982; 27:108–110. |
| 2. | 52/M | 12 mm | Glomus tumor (probably in the subcutaneous tissue) | Lawlor KB, Helm TN, Narurkar V, Vidimos A. Stump the experts. Multiple glomus Tumors. J Dermatol Surg Oncol. 1994; 20:629–630. |
| 3. | 57/F | Two nodular masses in the popliteal fat and other two between the hamstring muscle bellies. | Malignant glomus tumor | Gholve PA, Hosalkar HS, Finstein JL, Lackman RD, Fox EJ. Popliteal mass with knee pain in a 57-year-old woman. Clin Orthop Relat Res. 2007;457:253–259. |
| 4. | 17/M | 5 mm | Glomus tumor | Kawanami K, Matsuo T, Deie M, Izuta Y, Wakao N, Kamiya M, et al. An extremely rare case of a glomus Tumor in the popliteal fossa. J Orthop. 2016;13:313–315. |
| 5. | 9/F | 10×15×20 mm | Glomus tumor | Oztekin HH. Popliteal glomangioma mimicking Baker’s cyst in a 9-year-old child: an unusual location of a glomus tumor. Arthroscopy. 2003;19:19–23. |
| 6. | 51/F | 40 x 39.9 x42.6 mm | Glomus tumor | Present case |
Extradigital classical subcutaneous glomus tumor was detected on routine MR scan in our department. Single case of extra digital classical subcutaneous glomus tumour around the knee was detected on routine MR scan in our department in the last 2 years, who was a 24-year-old male patient presented with chronic pain on the anterior aspect of the knee [Figure 5].
![On axial proton density fat-saturated sequences, typical extradigital glomus tumor at the knee (a 24-year-old male patient presented with anterior knee pain, tenderness, and swelling) appears as well-circumscribed oval-shaped small homogenously hyperintense lesion (arrow) in superficial subcutaneous soft tissues. This is in contrast to our case which presented with infiltrative margins, deep location, large size, and heterogeneous mildly hyperintense T2 signals (as shown in Figure 2a and b) (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, interslice gap for axial PDFS 1.5 mm, TE - 51.1, TR – 3080).](/content/107/2020/2/1/img/IJMSR-2-082-g005.png)
- On axial proton density fat-saturated sequences, typical extradigital glomus tumor at the knee (a 24-year-old male patient presented with anterior knee pain, tenderness, and swelling) appears as well-circumscribed oval-shaped small homogenously hyperintense lesion (arrow) in superficial subcutaneous soft tissues. This is in contrast to our case which presented with infiltrative margins, deep location, large size, and heterogeneous mildly hyperintense T2 signals (as shown in Figure 2a and b) (acquisition protocol – 1.5 Tesla scanner [GE Signa HDxt], 4 mm slice thickness, interslice gap for axial PDFS 1.5 mm, TE - 51.1, TR – 3080).
The present case was atypical considering age, sex of the patient, its position, size, appearance, and MR morphology of the lesion. The large size of this glomangioma is rare, as mostly, tumors measure approximately 1–2 cm in size.[3] This tumor depicted irregular spiculated margins with uncommon feature of iso to slightly hyperintense signals on T2-W imaging, thus indicating cellular or solid variant of the tumor. There was the focal loss of fat planes with the biceps femoris muscle hence making it difficult to differentiate from soft-tissue sarcoma.
Soft-tissue sarcoma or synovial sarcoma was kept an important differential diagnosis in our case as it is the most common malignancy of the lower extremity in patients 6–35 years of age. The most frequent site of involvement is the popliteal fossa of the knee.[10] On MRI, synovial sarcoma typically shows “triple sign” on T2-W sequences, which represents intermixed areas of low (calcified or fibrotic collagenized regions), intermediate (a mixture of solid cellular elements), and high (hemorrhage or necrosis) signal intensity on T2-W images.[10] This typical imaging appearance of synovial sarcoma was not seen in the current case.
Other broad differential diagnoses include neural sheath tumor, particularly a localized neurofibroma; vascular tumor including hemangioma and hemangiopericytoma. In the current case, imaging findings were atypical and indeterminate; hence, a biopsy was planned to reach a conclusive diagnosis, which revealed a glomus tumor.
The clinical symptoms and imaging characteristics of a typical subungual mass usually provide adequate information for the diagnosis of glomus tumor; however, in the extra digital atypical locations, diagnosis can be challenging. Therefore, imaging suspicion for glomus tumors at uncommon sites is important. Surgical excision of glomus tumors is usually effective and curative in the patients.
CONCLUSION
Extradigital glomus tumors represent a rare etiology for soft-tissue mass, and the imaging findings are non-specific making pre-operative diagnosis difficult. High index of clinical suspicion, careful imaging, and early diagnosis can facilitate the treatment of these painful lesions. MRI characteristics, however, remain fairly consistent (relatively homogenous T1 iso-to-hypointensity and homogenous T2 hyperintensity), similar to the published literature and are similar to the more classic subungual location. This case highlights the prevalence of uncommon extradigital glomus tumors and without classic MRI morphology. Complete surgical excision with pathologic evaluation provides both definitive diagnosis and treatment.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
References
- Extradigital glomus tumors: A 20-year experience. Mayo Clin Proc. 2006;81:1337-44.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of glomus tumour on the knee: Case report and literature review. J Dermatitis. 2018;3:110.
- [CrossRef] [Google Scholar]
- Le glomus neuro-myo-artériel des régions tactiles et ses tumeurs. Lyon Chir. 1924;20:257-80.
- [Google Scholar]
- Popliteal glomangioma mimicking Baker's cyst in a 9-year-old child: An unusual location of a glomus tumor. Arthroscopy. 2003;19:19-23.
- [CrossRef] [Google Scholar]
- Characterisation of musculoskeletal tumours by multivoxel proton MR spectroscopy. Skeletal Radiol. 2017;46:483-95.
- [CrossRef] [PubMed] [Google Scholar]
- A new case of lower extremity glomus tumor up-to date review and case report. J Med Life. 2012;5:211-4.
- [Google Scholar]
- Extradigital glomus tumor in the knee: Excision with ultrasound guided needle localization. Skeletal Radiol. 2015;44:1689-93.
- [CrossRef] [PubMed] [Google Scholar]
- Glomangioma of the popliteal fossa. Apropos of a case. J Chir (Paris). 1997;134:436-7.
- [Google Scholar]
- Imaging of synovial sarcoma with radiologic-pathologic correlation. RadioGraphics. 2006;26:1543-65.
- [CrossRef] [PubMed] [Google Scholar]






