Translate this page into:
Unusual sites of giant cell tumor of bone

*Corresponding author: Suriyaprakash Nagarajan, Department of Radiodiagnosis, Government Medical College and Hospital, Tiruppur, Tamil Nadu, India. cbesuriya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nagarajan S, Kathirvelu G, Jaganathan D. Unusual sites of giant cell tumor of bone. Indian J Musculoskelet Radiol. 2023;5:128-34. doi: 10.25259/IJMSR_30_2022
Abstract
Giant cell tumor (GCT), a benign but regionally aggressive and destructive tumor, is most frequently found at the epiphysis of long bones. The distal femur, proximal tibia, and distal radius are the three most typical locations. The pelvis, proximal femur, proximal humerus, distal tibia, and sacrum are uncommon locations. About 18– 23% of benign bone neoplasms are GCT. They often manifest in early adulthood because they happen after the growth plate has closed, with the majority of instances being documented between the ages of 20 and 50. GCT is a primarily benign bone tumor that exhibits local recurrence, has metastasis potential, and may show malignant change. Depending on the site, complications such as hemorrhage or pathological fracture, GCTs can have varied appearances. Rarely, ribs, vertebral bodies, and bones of the hand and foot may be affected. Other than the long bones, the radiographic characteristics of GCT are non-specific and resemble those of other osteolytic diseases. This review shows GCT imaging features from both typical and unusual locales.
Keywords
Giant cell tumor
Giant cell tumor of hand
Magnetic resonance imaging
Subarticular lesions
INTRODUCTION
In 1818, Sir Astley Cooper published the first description of the giant cell tumor (GCT) of the bone.[1] The third decade of life has the largest incidence of GCT between the ages of 20 and 50, where 80% of cases occur.[2] GCT is best seen on radiographs in people with closed physes as a lytic, eccentric, and well-defined lesion with a non-sclerotic edge extending to the subchondral bone.[3] In this paper, we discuss imaging properties and normal and unusual GCT patterns.
CASE SERIES
Case 1
A 32-year-old lady had a painless growth of the left little finger that had been present for 6 months. A fusiform swelling was visible on the left little finger’s proximal phalanx on inspection [Figure 1a]. An expansile lytic lesion with a cortical break that spanned the whole proximal phalanx of the left little finger was visible on a radiograph of the left hand [Figure 1b]. The differential diagnosis at this time were metastases, Gorham disease, and GCT. Soft tissue lesion with cystic areas were noted involving the proximal phalanx of the left little finger on MRI [Figure 1c-f]. There was complete disintegration of the bone encasing the flexor tendons with preserved articular margins. The patient underwent ray amputation of the little finger and a specimen sent for histopathological examination. The proliferation of giant cells of the osteoclast type dispersed with mononucleated polygonal cells exhibiting fast mitotic activity at focal locations supported the diagnosis of a GCT [Figure 1g-h].
![(a) Fusiform swelling in the proximal phalanx of the left little finger. (b) Anterior-posterior radiograph of the hand shows an expansile lytic lesion involving the entire proximal phalanx with cortical destruction. The articular margins of metacarpophalangeal and proximal interphalangeal joints were intact (c) Axial T1-weighted image [T1WI], (d) Axial T2-weighted image [T2WI], (e) Coronal T2WI, and (f) Sagittal T2WI. Expansile T1 hypointense/T2 heterogeneously hyperintense soft-tissue lesion with multiple cystic spaces noted involving the proximal phalanx of the left little finger. Complete destruction of bone without extension to the articular surface and encasing the flexor tendons noted. Amputation of the left little finger was done under the supraclavicular block and sent for HPE. (g) High-power view of multinucleated osteoclast-type giant cells. (h) Post-operative image of the hand.](/content/107/2023/5/2/img/IJMSR-5-128-g001.png)
- (a) Fusiform swelling in the proximal phalanx of the left little finger. (b) Anterior-posterior radiograph of the hand shows an expansile lytic lesion involving the entire proximal phalanx with cortical destruction. The articular margins of metacarpophalangeal and proximal interphalangeal joints were intact (c) Axial T1-weighted image [T1WI], (d) Axial T2-weighted image [T2WI], (e) Coronal T2WI, and (f) Sagittal T2WI. Expansile T1 hypointense/T2 heterogeneously hyperintense soft-tissue lesion with multiple cystic spaces noted involving the proximal phalanx of the left little finger. Complete destruction of bone without extension to the articular surface and encasing the flexor tendons noted. Amputation of the left little finger was done under the supraclavicular block and sent for HPE. (g) High-power view of multinucleated osteoclast-type giant cells. (h) Post-operative image of the hand.
Only 2% of GCT are found in the hand, and because they recur there more quickly than they do elsewhere, they seem to be different from conventional GCT.[4] Surgery is the main therapeutic option, and the diagnosis is made using histological, radiological, and clinical data.
Case 2
A 36-year-old diabetic lady who had been complaining about left hip pain for 2.5 months arrived at the clinic. There was no history of trauma or loss of weight or loss of appetite or any localized signs of inflammation.
AP radiograph of the the pelvis showed an eccentric expansile lytic lesion in the greater trochanter of left femur with cortical thinning and narrow zone of transition. There was no matrix mineralisation [Figure 2a].
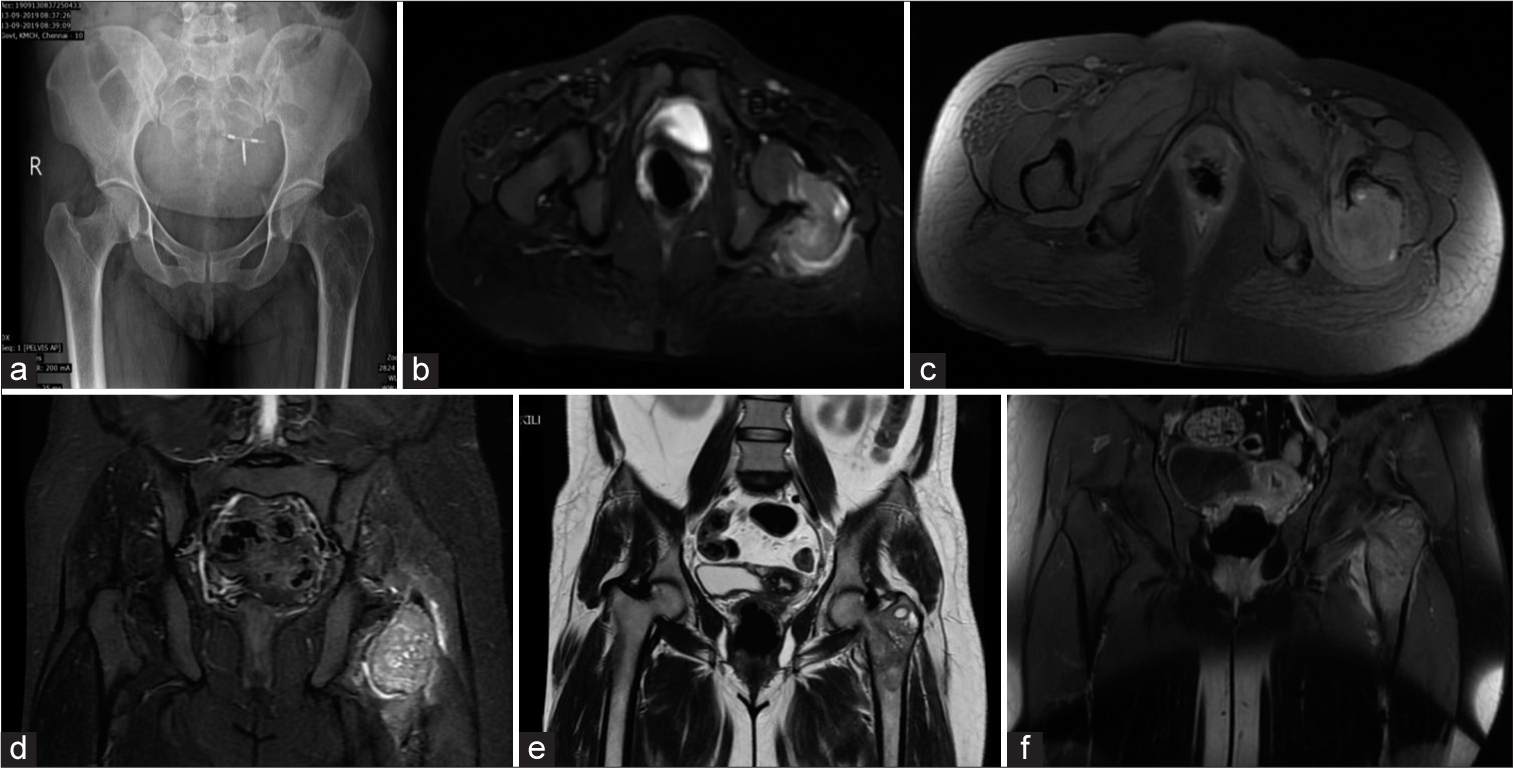
- (a) Frontal radiograph of pelvis showing an eccentric expansile lytic lesion with cortical thinning and narrow zone of transition in the greater trochanter of the left femur involving the neck of the femur not extending to the epiphysis. Note made of in situ intrauterine contraceptive device. (b) Axial short-tau inversion recovery (STIR) image showing hyperintense lesion extending in the neck of the femur. (c) Axial T1 WI with fat suppression showing hyperintense areas consistent with hemorrhagic foci. (d) Coronal STIR image showing hyperintensity in the muscle suggesting myofascial edema. (e) Coronal T2WI showing heterogeneously hypointense lesion with cystic areas in the meta-diaphyseal region of the proximal left femur involving the greater trochanter and neck. (f) Coronal T1 post-contrast image shows heterogeneous enhancement.
On MRI, there was an irregular, expansile lytic T1 hypointense/T2 heterogeneously hypointense/short-tau inversion recovery (STIR) hyperintense lesion with central, non-enhancing cystic areas in the greater trochanter, and proximal meta-diaphysis of the left femur [Figure 2b-e]. Heterogeneous enhancement was observed following the administration of contrast [Figure 2f]. Myofascial edema was noted adjacent to the lesion.
FNAC from the lesion revealed giant cells in a background of polymorphs, confirming the diagnosis of GCT, which is quite rare for this location.
Case 3
A 14-year-old female patient complained of recent swelling and soreness in the medial ankle [Figure 3a]. On examination, the medial right ankle joint had a tiny, firm, and sensitive swelling. An eccentric radiolucent lytic lesion involving the medial malleolus causing expansion and thinning of the cortex was seen on anteroposterior radiograph of the ankle [Figure 3b].
![(a) Swelling in the medial aspect of the right ankle. (b) Anterior-posterior (AP) radiograph of the right ankle shows an eccentrically located, radiolucent lesion involving the right medial malleolus. The lesion is well defined, lytic, with a thin sclerotic border with areas of cortical destruction. Periosteal reaction is not seen (c) Sagittal T1-weighted image [T1WI], (d) Coronal proton density fat-saturated sequence (PDFS), and (e) Sagittal T2-weighted image [T2WI]. A well defined T1 hypointense and T2/PDFS heterogeneously hyperintense lesion seen in the medial malleolus. The lesion is reaching up to the articular surface; however, no obvious extension is seen within the ankle joint. There is an extension into adjacent soft tissue. (f) Intraoperative image showing the bony lytic lesion. (g): AP and (h): Lateral view of the right ankle with bone cement and external fixator.](/content/107/2023/5/2/img/IJMSR-5-128-g003.png)
- (a) Swelling in the medial aspect of the right ankle. (b) Anterior-posterior (AP) radiograph of the right ankle shows an eccentrically located, radiolucent lesion involving the right medial malleolus. The lesion is well defined, lytic, with a thin sclerotic border with areas of cortical destruction. Periosteal reaction is not seen (c) Sagittal T1-weighted image [T1WI], (d) Coronal proton density fat-saturated sequence (PDFS), and (e) Sagittal T2-weighted image [T2WI]. A well defined T1 hypointense and T2/PDFS heterogeneously hyperintense lesion seen in the medial malleolus. The lesion is reaching up to the articular surface; however, no obvious extension is seen within the ankle joint. There is an extension into adjacent soft tissue. (f) Intraoperative image showing the bony lytic lesion. (g): AP and (h): Lateral view of the right ankle with bone cement and external fixator.
MRI was performed which showed a T1 hypointense lesion which was heterogeneously hyperintense on T2WI and proton density fat-saturated sequence (PDFS) [Figure 3c-e]. Few cystic areas were present within the lesion. There was minimal soft tissue extension along the medial aspect.
The patient underwent surgery, where extensive curettage and bone grafts were performed [Figure 3f-h]. Curette material was sent for histological examination, which confirmed GCT.
Case 4
A 19-year-old female patient arrived with a painless swelling at the lateral aspect of her right ankle. A well-defined, firm, and bony swelling that was non-adherent to the skin above was discovered on local examination.
An expansile lytic lesion of the meta-diaphyseal portion of the distal fibula including the lateral malleolus was visible on the right ankle’s radiograph and computed tomography (CT) [Figure 4a-c]. A lytic expansile solid lesion in the distal fibula was visible on the right ankle’s MRI, along with some localized cortical damage that extended upto the articular surface [Figure 4d-g]. The diagnosis of GCT was confirmed by HPE following an open biopsy.
![(a) Anterior-posterior radiograph, (b) Coronal computed tomography [CT] bone window, (c) Axial CT bone window. Lytic expansile lesion of the lateral malleolus with focal cortical break in the lateral aspect. No matrix calcification. No convincing periosteal reaction (d) Axial T1-weighted image, (e) Axial T2-weighted image, (f) Axial proton density fat-saturated sequence (PDFS), (g) Coronal short-tau inversion recovery [STIR]). The expansile solid lesion in the right distal fibula with some areas of focal cortical destruction extending upto the articular surface. The lesion displays an intermediate signal on T1/T2 and a hyperintense signal on STIR and PDFS. Edematous changes are seen in the perilesional soft tissues.](/content/107/2023/5/2/img/IJMSR-5-128-g004.png)
- (a) Anterior-posterior radiograph, (b) Coronal computed tomography [CT] bone window, (c) Axial CT bone window. Lytic expansile lesion of the lateral malleolus with focal cortical break in the lateral aspect. No matrix calcification. No convincing periosteal reaction (d) Axial T1-weighted image, (e) Axial T2-weighted image, (f) Axial proton density fat-saturated sequence (PDFS), (g) Coronal short-tau inversion recovery [STIR]). The expansile solid lesion in the right distal fibula with some areas of focal cortical destruction extending upto the articular surface. The lesion displays an intermediate signal on T1/T2 and a hyperintense signal on STIR and PDFS. Edematous changes are seen in the perilesional soft tissues.
GCT is known to occur in distal fibula in less than 1% cases.[5] A full surgical excision of all distal fibular GCT is required, followed by appropriate chemical cauterization and bone graft restoration. To have a good functional result, the ankle mortise must essentially be preserved.
Case 5
A 60 years old male had been complaining of right hip pain with limping for the past five 5 months. The right acetabulum, inferior pubic ramus, and ischium all exhibited cortical thinning and an ill-defined, expansile lytic lesion with a soap bubble appearance on the AP radiograph [Figure 5a]. On a CT scan, cortical discontinuity and the soft-tissue component were clearly visible [Figure 5b]. The diagnosis of GCT was supported by a biopsy.
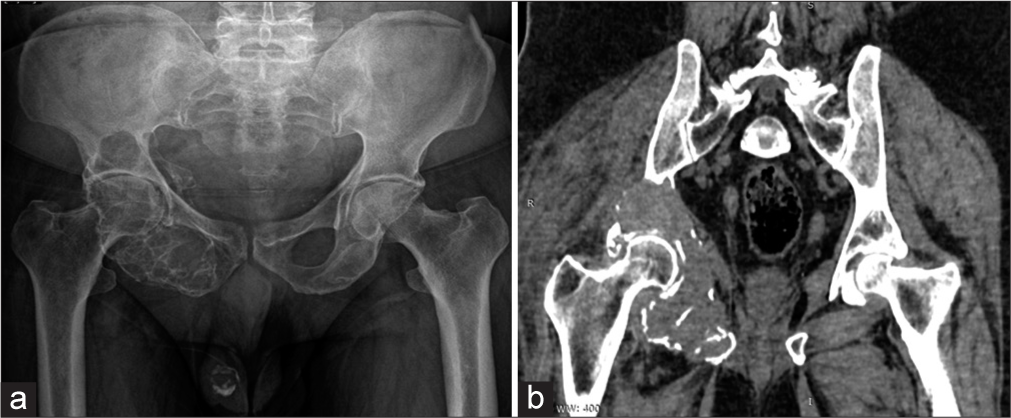
- (a) Anterior-posterior radiograph of pelvis, (b) Coronal computed tomography (CT) image. A multiloculated lytic lesion with sharp non-sclerotic margin seen in the right pelvis involving the acetabulum and ischium. No calcification and periosteal reaction. Soft-tissue component with cortical breach better demonstrated on CT.
GCTs of the pelvis can be found in the acetabular region, iliac region, and pubic and ischial regions, with the acetabular region being the most often affected area.[6] The diagnosis and treatment of pelvic GCT are particularly challenging due to the intricacy of the pelvic anatomy and the delayed onset of symptoms.
DISCUSSION
GCT is a locally aggressive benign bone tumor that affects the epiphyseal-metaphyseal region of the long bones and typically develops in young individuals with closed physes.[7] For a proper diagnosis, the patient’s age and the location of the tumor are necessary. Women are more affected by GCT than males and they are usually in the third or fourth decade. In patients with immature skeletons, the lesion is uncommon and unusual in patients under the age of 10. GCT of bone is regarded as benign; however, it is also well-known for its locally aggressive behavior, high rates of recurrence, and the potential for metastasis with a preference for the lungs.[8]
The main complaints are dull, agonizing pain, along with swelling and soreness. The distal femur, proximal tibia, distal radius, and proximal humerus are the areas of involvement that occur most frequently.
Radiography is the main method of GCT diagnosis. Staging, surgical planning, and lesion progression evaluation are frequently carried out using computed tomography imaging. MRI may be used to characterize tumors in more detail.[9]
GCT begins in the bone metaphysis and develops axially when the growth plate closes into the epiphysis until it reaches the area directly below the subchondral region [Figure 6a]. In radiography and CT scans, GCT is seen as a lytic lesion without internal matrix mineralization [Figure 6b]. The main distinguishing aspect of GCT is a narrow transition zone without a sclerotic border separating the tumor from the surrounding marrow. GCT structural heterogeneity results in several tissue interfaces that resemble fine internal septations [Figure 6c]. Imaging in GCT should include the entire bone to look for skip lesions [Figure 6d].
![(a) Anterior-posterior [AP] radiograph of wrist. Subchondral location of the tumor in the distal ulna (b) Sagittal computed tomography [CT] images of the left shoulder). The subarticular expansile geographic lytic lesion in the head of the humerus with cortical breach. The lesion extends to the adjacent soft tissues but not into the joint cavity. No internal matrix mineralization (c) AP radiograph of the wrist. An expansile eccentric lytic lesion with a soap bubble appearance, cortical thinning, and narrow zone of transition was noted in the distal radius (d) Sagittal T1WI of tibia. Imaging in GCT should include the entire bone to look for skip lesions (e) Axial CT bone window of distal femur. The expansile lytic lesion in eccentric location (f) Axial CT soft tissue window of proximal tibia – Cortical insufflation. (g) Axial T2WI of the knee – T2 heterogeneously hyperintense subarticular well-defined eccentric lesion with severe cortical thinning and cortical break along the anterior aspect. Few fluid levels are noted.](/content/107/2023/5/2/img/IJMSR-5-128-g006.png)
- (a) Anterior-posterior [AP] radiograph of wrist. Subchondral location of the tumor in the distal ulna (b) Sagittal computed tomography [CT] images of the left shoulder). The subarticular expansile geographic lytic lesion in the head of the humerus with cortical breach. The lesion extends to the adjacent soft tissues but not into the joint cavity. No internal matrix mineralization (c) AP radiograph of the wrist. An expansile eccentric lytic lesion with a soap bubble appearance, cortical thinning, and narrow zone of transition was noted in the distal radius (d) Sagittal T1WI of tibia. Imaging in GCT should include the entire bone to look for skip lesions (e) Axial CT bone window of distal femur. The expansile lytic lesion in eccentric location (f) Axial CT soft tissue window of proximal tibia – Cortical insufflation. (g) Axial T2WI of the knee – T2 heterogeneously hyperintense subarticular well-defined eccentric lesion with severe cortical thinning and cortical break along the anterior aspect. Few fluid levels are noted.
When compared to the central axis of the bone, GCTs typically develop at an eccentric location [Figure 6e]. The whole medullary cavity is occupied by the lesions in large tumors and those growing in small bones, making it impossible to pinpoint their origin. GCT’s expansional nature causes the cortex to weaken and degrade, which results in different degrees of cortical insufflation [Figure 6f]. Cortical erosion, periosteal reaction, soft-tissue mass, and joint invasion are signs of the tumor’s aggressive character. The edema of the surrounding soft tissues and bone marrow appears as hyperintensity in T2WI and hypointensity in T1WI.
The tumors’ intramedullary component will be best assessed on T1WI and its component outside the bone is properly assessed on T2WI. Definitive diagnosis must be correlated by three means – radiological [Table 1], clinical, and histological.
| Radiograph | CT | MRI |
|---|---|---|
| Primary diagnosis modality FINDINGS
|
|
Tumor characterization
|
GCT: Giant cell tumor, T1WI: T1 Weighted image, T2WI: T2 Weighted image
Pathological fracture and secondary aneurysmal bone cyst transformation with fluid levels [Figure 6g] are the most common complications of the GCT of bones.[10] The best course of treatment for GCT is complete en bloc excision of the tumor. Most often, curettage is used to treat GCT, followed by the insertion of cement and thorough follow-up to check for any new lytic regions at cement-bone interfaces. Better examination of lytic regions and recurrent soft-tissue masses is made possible by CT and MRI.
Despite being a quasi-malignant tumor, GCT can spread to the lungs.[8] Although observation of pulmonary metastasis may be the first line of management, this may not be the rule in every case metastasectomy, chemotherapy and symptomatic treatment are among other options available.
The presence of multinucleated giant cells in the HPE is diagnostic of giant cell tumors. However, if there is a clinical suspicion of hyperparathyroidism, IHC analysis may be indicated. In our case series, since the blood parameters like serum calcium were normal, the possibility of PHT was ruled out.
CONCLUSION
The imaging characteristics of GCT and other tumor mimics are very similar. Clinicoradiologic correlation and histological confirmation with core-needle or open biopsy are required for an accurate diagnosis. A more aggressive benign tumor-like GCT should be considered if an obvious expansile lesion is present in an unusual location. Sclerotic margin, calcification matrix, and periosteal response are some unusual ways that GCT might show. To look for skipped lesions during GCT imaging, the complete bone should be scanned.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The two faces of giant cell tumor of bone. Cancer Lett. 2020;489:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of bone: Review, mimics, and new developments in treatment. Radiographics. 2013;33:197-211.
- [CrossRef] [PubMed] [Google Scholar]
- Rare site giant cell tumors: Report of two cases on phalanges of the finger and review of literature. J Orthop Traumatol. 2009;10:193-7.
- [CrossRef] [PubMed] [Google Scholar]
- Saving the ankle in distal fibular giant cell tumour-a case report. J Clin Orthop Trauma. 2019;10:1054-8.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the pelvis: A systematic review. Orthop Surg. 2015;7:102-7.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of bone. Prognosis and treatment of pulmonary metastases. Clin Orthop Relat Res. 1997;338:205-14.
- [CrossRef] [PubMed] [Google Scholar]
- Description of Radiological Changes of Giant Cell Tumor of Bone after Treatment with Denosumab In: Osteosarcoma like Appearance. ECR 2019 EPOS. European Congress of Radiology-ECR 2019. Available from: https://epos.myesr.org/poster/esr/ecr2019/C-3326 [Last accessed on 2021 May 19]
- [Google Scholar]
- Giant cell tumour of bone: A new evaluating system is necessary. Int Orthop. 2012;36:2521-7.
- [CrossRef] [PubMed] [Google Scholar]






