Translate this page into:
An atypical presentation of Ewing’s sarcoma of the scapula in an elderly male: A case report

*Corresponding author: Priyadharshini Bargunam, Department of Pathology, Vardhman Mahavir and Safdarjung Hospital, New Delhi, India. priyasweetygunam@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shaikh S, Singh U, Saifi AS, Bargunam P. An atypical presentation of Ewing’s sarcoma of the scapula in an elderly male: A case report. Indian J Musculoskelet Radiol. doi: 10.25259/IJMSR_34_2024
Abstract
Ewing’s sarcoma (ES) is a malignant bone tumor typically found in children, with a rare incidence in adults. This case highlights an unusual occurrence of ES in a 54-year-old male who presented with pain and swelling in his left scapula. Imaging showed a highly aggressive bone tumor with metastasis to the lungs. A definitive diagnosis was established through histopathology and immunohistochemical (IHC) studies, and the patient has since responded positively to chemotherapy. While ES in the scapula is exceedingly rare – fewer than 80 cases have been reported – the disease often can remain undetected until it reaches advanced stages. Diagnosis hinges on detailed histological and IHC analysis, and treatment generally includes chemotherapy, with surgery or radiotherapy based on tumor characteristics. This case emphasizes the need to include ES as a potential diagnosis in older patients with scapular tumors, even though it is typically seen in younger individuals.
Keywords
Sarcoma
Scapula
Ewing’s
Neoplasm
Bone tumor
Metastasis
INTRODUCTION
Metastasis is the most common type of malignant bone tumor across all age groups. Osteosarcoma, Ewing’s sarcoma (ES), and chondrosarcoma are the most prevalent primary malignant bone tumors. Stout described an undifferentiated round cell tumor in 1918 following which James Ewing in 1921 first described ES as an undifferentiated tumor arising from mesenchymal cells within, and our understanding of the disease has developed significantly since then.[1]
ES, a malignant tumor originating from primitive neural cells, is one of the most frequent bone tumors in children and younger adults.[2] ES constitutes 3% of all pediatric cancers and around 10% of primary malignant bone tumors, with its highest incidence during the early twenties.[3] ES develops predominantly in the diaphysis and meta-diaphyseal region of long bones such as the femur, humerus, and tibia. However, it is also frequently seen in flat bones like ilium. The tumor infrequently affects the skull, vertebral bones, phalanges, Carpals, metacarpals, tarsals, metatarsals, or the scapula.
Here, we report a rare case of ES in the scapula of an elderly man. With fewer than 80 cases of scapular ES documented in the literature, this report contributes to the limited knowledge of ES in this uncommon location.
CASE REPORT
A 54-year-old male presented to the outpatient department with a 3-month history of pain and swelling in the left scapular region. The pain, which initially occurred only with arm movement, gradually worsened and became constant, unresponsive to medication. Clinical examination revealed a well-defined, hard, non-mobile, non-tender, round swelling measuring approximately 5 × 5 cm, with a localized temperature increase. There was no distal neurovascular deficit but he reported significant weight loss, fatigue, and a low-grade fever.
A plain radiograph of the left shoulder joint revealed a single permeative lytic lesion with ill-defined margins, a wide zone of transition, and no periosteal reaction in the scapula. The lesion had a significant extraosseous soft-tissue component, displacing adjacent fat pads and having no internal mineralized matrix. Multiple metastatic foci were identified in the visualized hemithorax [Figure 1]. Magnetic resonance imaging (MRI) showed involvement of the left glenohumeral and acromioclavicular joints, as well as pulmonary metastases [Figures 2a and 2b]. Dynamic contrast-enhanced MRI confirmed the same and the Ktrans/apparent diffusion coefficient (ADC) ratio came out to be 2.59 (Reference- 0.125) [Figure 2c]. Positron emission tomography (PET) and computed tomography (CT) demonstrated a metabolically active expansile lesion in the left scapula with both metabolically active and inactive pulmonary lesions, patchy marrow-based lesions in the dorsolumbar vertebrae, and focal uptake in the prostate.
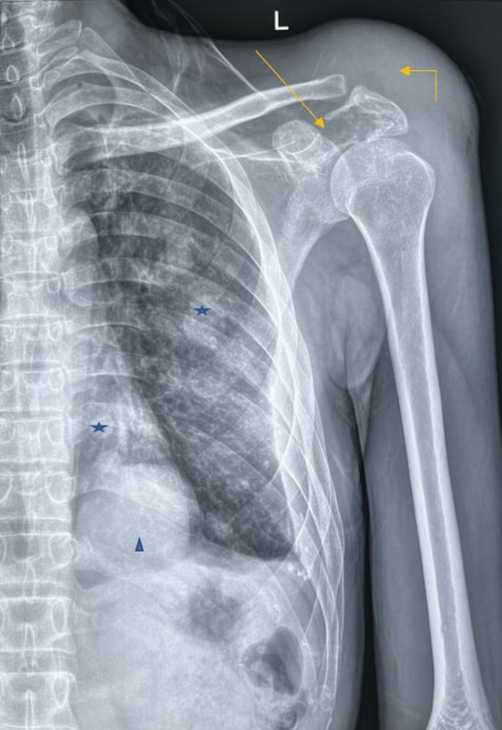
- Anteroposterior view radiograph of the right shoulder joint in a skeletally mature adult demonstrates a destructive lytic lesion centered in the left scapular spine and acromion (arrow). This lytic lesion shows ill-defined non-sclerotic margins with a wide zone of transition, no periosteal reaction, and a significant extraosseous soft-tissue component (elbow arrow) protruding superiorly displacing the adjacent fat pads. No internal mineralized matrix is appreciable. Of note, multiple variable-sized well defined round-oval radio-opaque masses are seen randomly distributed in the left hemithorax (star) with the dominant mass overlying the cardiac silhouette (arrowhead).
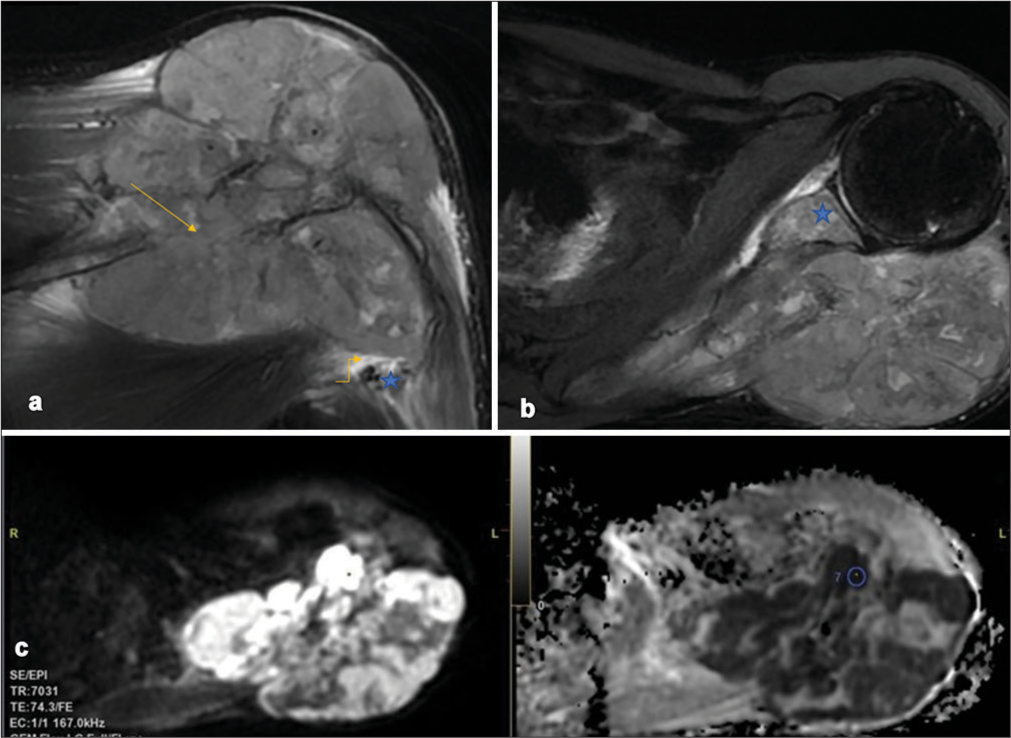
- (a) Coronal T2-weighted (T2W)-fat-saturated image demonstrates an intraosseous bony lesion (arrow) in the scapular spine with significant soft-tissue causing bony destruction and spread into adjacent soft tissues. The axillary neurovascular (star) bundle along the inferolateral aspect of the bone primary is devoid of any tumor contact with no abutment/encasement (elbow arrow). (b) Axial T2W-fat saturated image demonstrates intra-osseous spread into the glenoid (star) and its subchondral aspect signifying glenohumeral joint involvement. The mass demonstrates no areas of osseous/chondroid/adipocytic/fibrous/hemorrhagic matrix. (c) Diffusion-weighted imaging (b-value 800s/mm2) and corresponding ADC map show the mass predominantly diffusion restricting with a minimum ADC in the most restricting component of 0.227 × 10−3mm2/s.
An ultrasound-guided biopsy was performed. Histopathological examination revealed sheets of uniform small round tumor cells with round nuclei, stippled chromatin, inconspicuous nucleoli, and scant to clear eosinophilic cytoplasm, with 1–2 mitoses per 10 high power fields and few necrotic areas [Figure 3a-c]. Immunohistochemistry showed diffuse membranous positivity for CD99 and diffuse nuclear positivity for Homeobox protein Nkx-2.2 (NKX2.2), with 30–40% positivity for ETS translocation variant 4 (ETV4) and a Ki67 proliferation index of 30–35% [Figures 3 d-g]. Tumor cells were negative for CD45, S100, special AT-rich sequence-binding protein 2 (SATB2), avian v-ets erythroblastosis virus E26 oncogene homolog (ERG), Friend leukemia integration 1 (FL1), CD34, synaptophysin, and BCL6 corepressor (BCOR) [Figure 3h]. Based on these findings, a final diagnosis of ES of the left scapula was made.
![(a-c) Mildly pleomorphic small round blue cells with scant cytoplasm, irregular hyperchromatic nucleus, and inconspicuous nucleoli. Cytoplasmic clearing is appreciated in 50–60% of cells. (a- Hematoxylin and Eosin [H&E], ×10; b and c- H&E, ×40); (d) The tumor cells show diffuse membranous positivity with CD 99 (×40); (e) Diffuse nuclear positivity with NKX2.2 (×40); (f) Nuclear positivity with ETV4 (×40); (g) Ki 67 proliferative index was 30–35% (×40); (h) The tumor cells were negative with BCOR (×40).](/content/107/2024/0/1/img/IJMSR-34-2024-g003.png)
- (a-c) Mildly pleomorphic small round blue cells with scant cytoplasm, irregular hyperchromatic nucleus, and inconspicuous nucleoli. Cytoplasmic clearing is appreciated in 50–60% of cells. (a- Hematoxylin and Eosin [H&E], ×10; b and c- H&E, ×40); (d) The tumor cells show diffuse membranous positivity with CD 99 (×40); (e) Diffuse nuclear positivity with NKX2.2 (×40); (f) Nuclear positivity with ETV4 (×40); (g) Ki 67 proliferative index was 30–35% (×40); (h) The tumor cells were negative with BCOR (×40).
The patient is currently undergoing chemotherapy with Doxorubicin, and Cyclophosphamide and is responding well to treatment. Informed consent was obtained for reporting this case.
DISCUSSION
ES commonly occurs in the pelvis and lower extremities but rarely in the scapular body. Fewer than 80 cases of ES of the scapula have been documented in the literature.[4] The scapula is a rare location for bone tumors, representing only 3% of cases, with the majority being malignant. Chondrosarcoma and osteosarcoma are the most frequently occurring tumors in the scapula.
The occurrence of ES decreases notably after the age of 20, and in older patients, treatment becomes more challenging due to a lower response to chemotherapy and higher drug toxicity.
Scapular ES is more frequently seen in males, with ages ranging from 41 to 86 years (median age of 53) with three cases of congenital ES reported involving the shoulder girdle.[5] Our patient fell within this age bracket. He experienced ongoing shoulder pain that ranged from dull to severe, progressively worsening without any neurovascular deficits. On physical examination, a firm mass in the scapular area was typically observed. The primary symptoms included pain and a palpable mass, with potential accompanying signs such as fever, anemia, leukocytosis, and increased erythrocyte sedimentation rate (ESR) levels.[6]
The exact cause of ES is still unclear, but most cases involve a cytogenetic translocation, featuring rearrangements between the Ewing Sarcoma (EWS) gene and a member of the E26 transformation specific (ETS) gene family, typically FLI1, located on chromosome 22q12. Radiologically, ES is seen as a poorly defined osteolytic lesion with permeative or “worm-eaten” bone destruction, often accompanied by a multilayered periosteal reaction resembling onion skinning.[1] On MRI, the lesion commonly appears as a prominent mass with regions of necrosis or hemorrhage.[7]
The most commonly used treatment protocol for ES is an intense multi-agent neoadjuvant chemotherapy, followed by en bloc excision of the tumor mass and post-operative radiotherapy if there is doubt of tumor residue. ES of the scapula with metastatic disease is treated with marginal resection.[8]
In this case, the initial radiological differentials included were metastasis and an aggressive chondroid lesion. Histopathological differentials included small-cell osteosarcoma, mesenchymal chondrosarcoma, lymphoma, and metastatic neuroblastoma. These were ruled out through immunohistochemical tests: Tumor cells were negative for CD45 (excluding lymphoma) and negative for S100, synaptophysin, and chromogranin (neuroendocrine).
The cells were also negative for SATB2, ERG, FLI1, CD34, and BCOR, excluding small-cell osteosarcoma. PET-CT scans showed no increased uptake in other organs apart from the lungs and scapula. Local invasion is found in 63.64% of cases, while metastases occur in 35.71% of cases,[6] most commonly involving the lungs, as observed in our patient. Lymph nodes, bones and bone marrow, and spinal intradural spaces are other areas involved.[4]
ES is sensitive to radiation, and radiotherapy can be employed preoperatively, postoperatively, or as the primary treatment when surgery is not feasible. Without multi-agent chemotherapy, the 5-year survival rate for ES is under 10% with only surgery or radiotherapy. However, this improves to 60–70% for localized disease and 20–40% for metastatic cases when multi-agent chemotherapy is used.[9] Table 1 presents various studies on scapular ES along with their findings.
| Author | Year | Age | Sex | Presentation | Treatment and response |
|---|---|---|---|---|---|
| Mavrogenis et al.[4] | 2009 | 57 | Male | Scapular ES with pulmonary Metastasis | Scapulectomy and survived for 12 months |
| Hiramoto et al.[9] | 2013 | 65 | Female | Scapular ES following chemotherapy to diffuse large B cell lymphoma | Died after 2 years of chemotherapy due to pulmonary metastasis |
| Malik et al.[8] | 2020 | 51 | Male | Scapular ES with multiple metastases | Death due to the disease |
| Malik et al.[8] | 2020 | Between 1988 and 2008, 29 Scapular ES (14 females and 15 males) from a single institution in UK | The disease-specific survival rate for all patients was 71.4% at 5 years and 63% at 10 years. For those who had surgery, the survival rate improved to 86.5% at 5 years and 81% at 10 years. | ||
| Shashaa et al.[6] | 2022 | Review of 15 studies on scapular ES | ES was most commonly observed in Asia, with a higher prevalence in males (60%; 3:2 ratio). The primary symptom was swelling, reported in 73.33% of cases. Diagnostic methods included plain radiography in 46.6% of cases, CT scans in 60%, and immunohistochemistry in 86.6%. Local invasion was present in 63.64% of cases, while metastases occurred in 35.71%. | ||
| This case | 2024 | 54 | Male | Scapular ES with Pulmonary Metastasis | Undergoing neoadjuvant chemotherapy and responding well |
ES: Ewing’s sarcoma, CT: Computed tomography
CONCLUSION
ES of the scapula is a common tumor in an uncommon location. In adults, the prognosis is poorer, making it crucial to consider this in the differentials of scapular tumors.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in editing of the manuscript.
Financial support and sponsorship
Nil.
References
- Enzinger and Weiss’s soft tissue tumors E-book Netherlands: Elsevier Health Sciences; 2019.
- [Google Scholar]
- Extraskeletal Ewing’s sarcoma of the scapula: A case report and literature review. J Med Assoc Thai. 2018;101:S185-8.
- [Google Scholar]
- Ewing sarcoma family of tumors. J Am Acad Orthop Surg. 2010;18:94-107.
- [CrossRef] [PubMed] [Google Scholar]
- Total scapulectomy and constrained reverse total shoulder reconstruction for a Ewing's sarcoma. J Surg Oncol. 2009;100:611-5.
- [CrossRef] [PubMed] [Google Scholar]
- Eight-year follow-up after scapulectomy in a neonate with congenital Ewing sarcoma of the scapula. J Shoulder Elbow Surg. 2018;27:e288-93.
- [CrossRef] [PubMed] [Google Scholar]
- Ewing's sarcoma in scapula, epidemiology, clinical manifestation, diagnosis and treatment: A literature review. Ann Med Surg. 2022;77:103617.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics and oncologic outcomes of patients with Ewing sarcoma of the scapula. Surg Oncol. 2021;38:101619.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of Ewing's sarcoma of the scapula-a long-term follow-up study. Orthop Traumatol Surg Res. 2020;106:25-30.
- [CrossRef] [PubMed] [Google Scholar]
- Ewing sarcoma arising after treatment of diffuse large B-cell lymphoma. JPN J Clin Oncol. 2013;43:417-21.
- [CrossRef] [PubMed] [Google Scholar]







